Figures & data
Table 1. Specific issues in the toxicity testing of NPs.
Figure 1. Extent of NP exposure, translocation and use of advanced cell culture models in the testing for epithelial barriers and internal organs. Independent from the extent of exposure use of in vitro models for protective barriers (cornea, epidermis, oral cavity, vaginal epithelium) is low as good ex vivo systems are available. In vitro systems are used when particle exposure is high and robust ex vivo systems are missing (alveolar and intestinal epithelium).
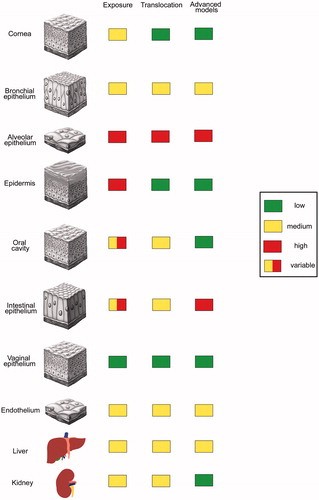
Table 2. Origin and use of cell lines in the physiologically relevant models.
Figure 2. Use of transwell membranes in advanced culture models. Monoculture for permeation experiments (A), indirect contact (B) and direct or indirect contact (C) co-culture of only one cell type in each chamber. Cells can be cultured or separated by matrices that may either be acellular (D) or contain one (E) or several types of cells (E, F). Co-culture systems may consist of two and more cell types in the apical compartment (G), co-culture of two and more cell types in the apical compartment in indirect culture with one cell type in the basolateral compartment (H), co-culture of one cell type in the apical and several types of cells in the basolateral compartment (I), combined direct contact and indirect contact culture (J), direct contact culture of several cell types in the apical compartment and one type in the basolateral compartment (K). The separation line in H, J and K indicates that different cell types in monoculture can be used in the basolateral compartment or in the apical compartment (E).
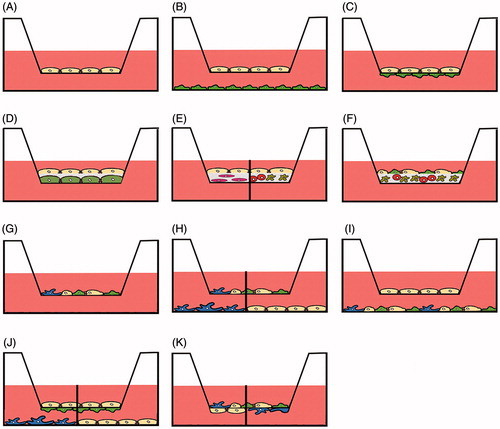
Figure 3. Particle and biological parameter that were identified to play a role in in silico modelling of metal oxide NPs (according to the meta-analysis by Ha et al. [Citation105]). Parameters can be influenced by the use of advanced cell culture models, either by medium composition (M) or by the culture method (C). Medium composition may have an influence on aggregation (hydrodynamic size) and influence the dose that reaches the cell. In addition, surface parameters may be changed. The culture method influences mainly cellular differences by increasing cell differentiation and the exposure time as physiologically relevant culture methods usually enable exposure for longer time periods.
![Figure 3. Particle and biological parameter that were identified to play a role in in silico modelling of metal oxide NPs (according to the meta-analysis by Ha et al. [Citation105]). Parameters can be influenced by the use of advanced cell culture models, either by medium composition (M) or by the culture method (C). Medium composition may have an influence on aggregation (hydrodynamic size) and influence the dose that reaches the cell. In addition, surface parameters may be changed. The culture method influences mainly cellular differences by increasing cell differentiation and the exposure time as physiologically relevant culture methods usually enable exposure for longer time periods.](/cms/asset/4b39befd-93dc-41e9-80f8-9a0d8c3e1291/ianb_a_1479709_f0003_c.jpg)
Table 3. Parameters included in QSAR models.
