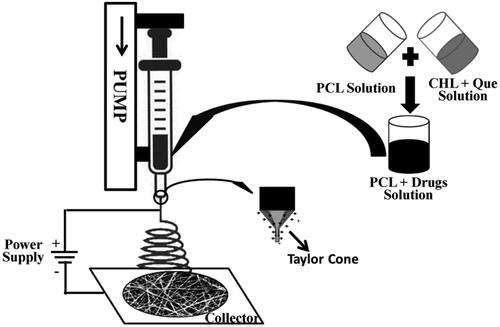
Figures & data
Table 1. Fibre morphology, average diameter, porosity and entrapment efficiency of nanofiber membrane of different compositions.
Table 2. ‘Time-dependent’ DPPH scavenging efficacy of ciprofloxacin HCl and quercetin loaded nanofiber in PBS (pH 7.4).
Figure 2. HR-SEM images of electrospun nanofiber: F1- PCL nanofiber (8% w/v), F2- PCL nanofiber (12% w/v), F3- PCL/CHL nanofiber, and F4- PCL/CHL/Que nanofiber.
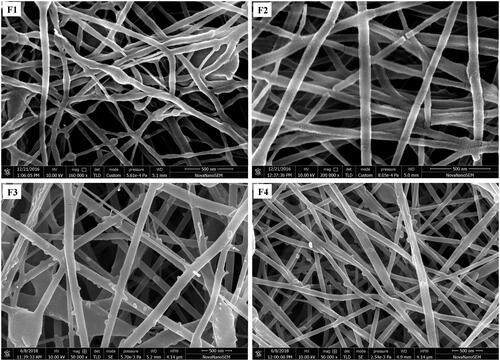
Figure 3. Two dimensional and three dimensional morphology of PCL/CHL/Que nanofiber showing bead-free and continuous nanofiber.
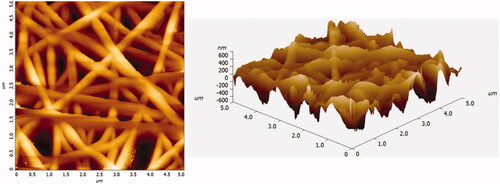
Figure 4. In-vitro cumulative drug release profiles of ciprofloxacin HCl and quercetin loaded nanofiber in PBS (pH 7.4). The data are represented as mean ± SD, n = 3.
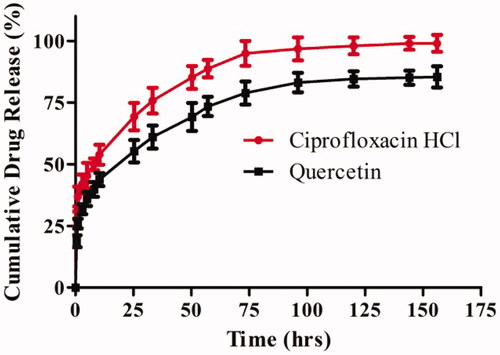
Figure 5. DPPH scavenging activity of nanofiber on ‘fixed reaction time’: (i) UV-visible spectra of different DPPH solution after 30 min incubation; (ii) bar graph comparing the scavenging efficacies of different nanofiber. ap < .05 compared to PCL-NF; bp < .05 compared with PCL/CHL-NF. The data represented as mean ± SD, n = 3.
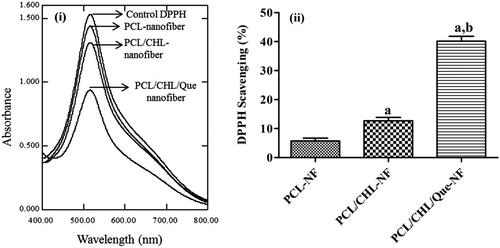
Figure 6. Agar plate showing the growth inhibition zone of S. aureus on (i) day 1, and (ii) day 7. F2, F3, and F4 represent PCL, PCL/CHL, and PCL/CHL/Que loaded nanofiber. Graph (iii) outline the relation between inhibition zone (mm) and time (days). The data represented as mean ± SD, n = 3.
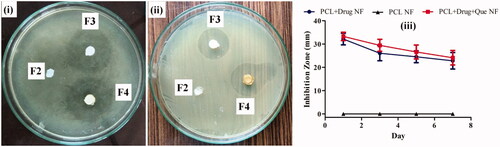
Figure 7. Biocompatibility of fabricated scaffold: (i) haemolysis activity of different nanofiber. ap < .05 compared to PCL-NF; bp < .05 compared with PCL/CHL-NF, and (ii) viability of fibroblast cells after treatment with the extract of different nanofiber. *p < .05 versus control. The data are represented as mean ± SD, n = 3.
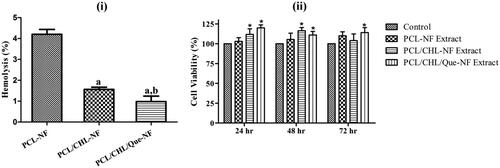
Figure 8. In-vivo healing of full thickness wound: (i) images of wounds, and (ii) wound area closure rate after treatment with gauze, PCL, PCL/CHL, PCL/CHL/Que-nanofiber at different time interval. *p < .05, **p < .01 and ***p < .001 versus gauze treated group; #p < .05, ##p < .01 and ###p < .001. The data are expressed as means ± SD, n = 6.
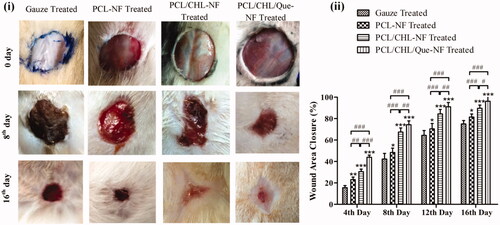
Figure 9. Haematoxylin − eosin stained slice showing histological changes in granulation tissue after treatment with gauze, PCL-NF, PCL/CHL-NF and PCL/CHL/Que-NF.
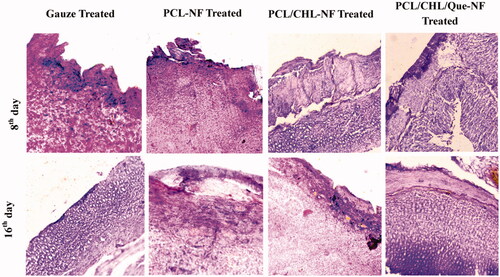
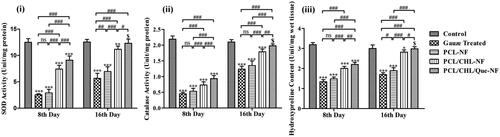
Figure 10. Biochemical assessment of granulation tissue harvested from wound area in terms of (i) SOD, (ii) catalase activity, and (iii) hydroxyproline content on day 8th and 16th. *p < 0.05, **p < .01, ***p < .001, and $p > .05 versus gauze treated group; #p < .05, ##p < .01, ###p < .001 and nsp > .05. The data are expressed as means ± SD, n = 6. Control is an unwounded group.