Figures & data
Figure 1. Molecular biology analysis of decellularized porcine dermis. (a) Histological and DAPI stains show the preserved ECM without residual cells. (b) Extracted porcine DNA was quantified and qualified. (c) The major components of ECM—collagen, GAG, and elastin—were preserved in the dECM.
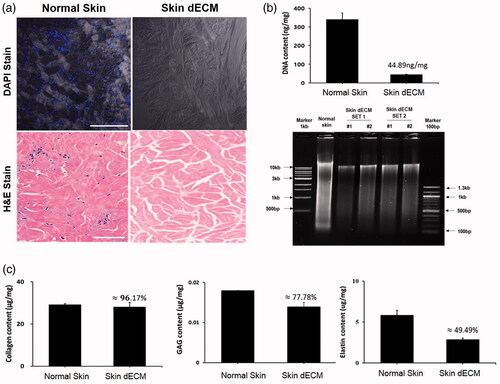
Figure 2. Residual ECM and growth factors in skin dECM. (a) The LC/MS assay showed that ECM components were preserved and certain cellular components were removed by the decellularization process. (b) The remaining growth factors in skin dECM and collagen were analysed using the Quantibody array. Among the 40 growth factors, 24 growth factors were found at a higher degree than collagen. Schematic figure of Quantibody array presented by RayBiotech.
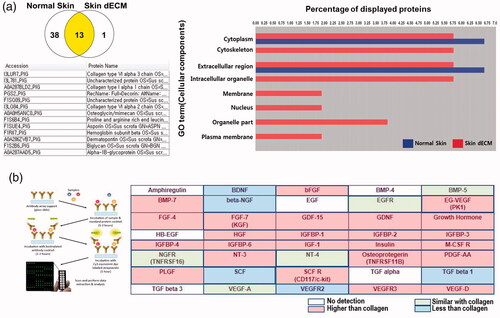
Figure 3. Survival and proliferation of HDFs in the 3D printed cell laden construct. (a) Structure of the 3D printed construct using skin bio-ink involved layer by layer stacking. (b) HDFs survived and proliferated in the constructs as well as maintained their structure.
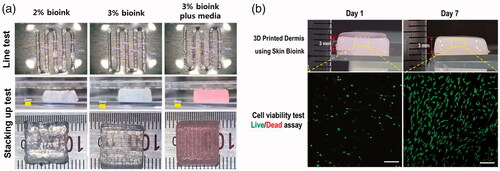
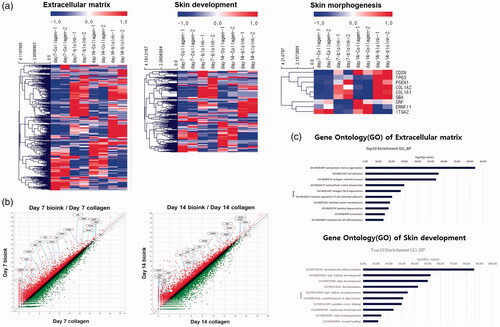
Figure 4. Changes in gene expression in the 3D printed cell laden construct. (a) The heatmap shows that genes related to ECM, skin development, and skin morphology were increased in the 3D printed cell laden construct. (b) The scatter plots show that ECM and ECM-related genes in the skin bio-ink were upregulated. (c) Gene Ontology for ECM and skin development shows that skin development was enriched in the 3D printed cell laden construct.