Figures & data
Figure 1. (A) Transmission electron microscope images of apigenin loaded nanotransfersome and (B) Con-A conjugated nanotransfersomal gel, (C) Mean vesicle size of nanotransfersomes, (D) Mean vesicle size of Con-A conjugated nanotransfersomal gel, (E) Zeta potential of nanotransfersomes and (F) Zeta potential of Con-A conjugated nanotransfersomal gel.
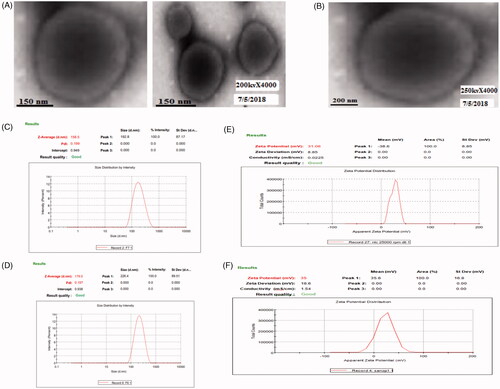
Table 1. Zeta potential, vesicles size and PDI of optimized formulations.
Figure 2. (A) X-ray diffractogram of (a) Apigenin, (b) Con-A, (c) Phospholipids, (d) physical mixture, (e) Con-A conjugated nanotransfersomal gel. (B) FTIR spectra of (a) Apigenin, (b) Con-A, (c) Con-A conjugated nanotransfersomal gel. (C) DSC Thermogram of (a) Apigenin (b) Con-A conjugated nanotransfersomes (c) Con-A (d) Phospholipids (E) Carbodiimide. (D) In vitro drug release profile of Con-A conjugated nanotransfersomal gel, marketed product and pure drug suspension in skin pH 5.5.
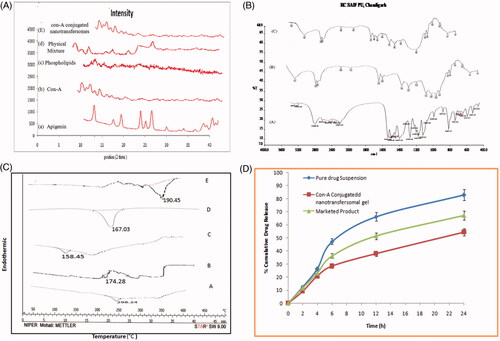
Table 2. In vitro drug release profile of Con-A conjugated nanotransfersomal gel, marketed product and pure drug suspension in skin pH 5.5.
Table 3. Absorbance data for the interference of additives in the estimation of apigenin.
Table 4. Release behaviour of apigenin from Con-A conjugated nanotransfersomal gel formulations.
Table 5. Permeation and % drug retention data for apigenin loaded Con-A conjugated nanotransfersomal gel, marketed and pure drug suspension across abdominal goat skin.
Table 6. Effect of apigenin, unloaded and apigenin-loaded Con-A conjugated nanotransfersomal gel on cellular viability (as % of control) of MTT assay on (a) HaCaT cells and (b) A375 cells.
Figure 3. (A) (a) Showing the photomicrograph of the Mice skin of control group, (b) UV-Irradiated Mice skin of control group, (c) UV-Irradiated Mice skin of PDS group, (d) UV-Irradiated Mice skin of marketed formulation (Flonida, Shalak Pharmaceutical India) group, (e) UV- Irradiated Mice skin of Con-A Conjugated NTG group. (B) Showing the effect of all prepared formulations on the level of increase of MPO. (C) CLSM image showing the depth of skin penetration of Rhodamine red (florescence marker) after 24 h into the goat skin surface at 250 µm (a) (PDS), (b) MP, (c) CCNTG. (D) effect of apigenin, unloaded and apigenin-loaded Con-A conjugated nanotransfersomal gel on cellular viability (as % of control) of MTT assay on HaCaT Cells (a) and A375 cells (b).
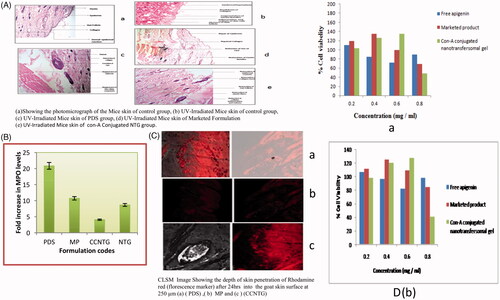
Table 7. Storage stability studies of the Con-A conjugated nanotransfersomal gel at different time interval and temperature conditions.
