Figures & data
Figure 1. CircCDYL is derived from the exon 4 (667 bp) of CDYL and formed by a back-splice event. (A) Schematic illustration showed the sequence of CDYL gene, and two primate-specific Alu elements were in an inverted orientation in the flanking introns of CDYL exon4. (B) Blast alignment showed the highly reverse complement of the inverted repeated Alu elements. (C) The existence of circCDYL was validated in two bladder cancer Cell lines EJ and T24T by RT–PCR. β-actin was used as negative control. (D) The head-to-tail splicing in the RT–PCR products of circCDYL with expected size were confirmed by Sanger sequencing. Red arrow represents ‘head-to-tail’ splicing sites of circCDYL.
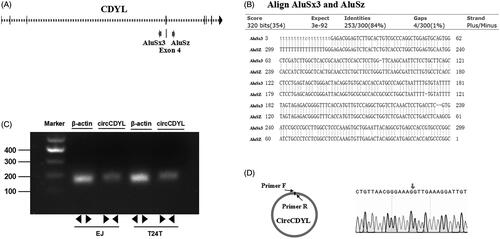
Figure 2. The expression and subcellular location of circCDYL in human bladder cancer tissues and cell lines. (A) The expression of circCDYL was detected by quantitative real-time PCR in 30 pairs of bladder cancer tissues and normal bladder tissues. GAPDH was used as a negative control. Data are mean ± SEM, n = 3. **p < .05 (Student’s t-test). (B) The expression of circCDYL was tested by quantitative real-time PCR in SV-HUC-1, EJ and T24T cells. GAPDH was used as internal control. Data are mean ± SEM, n = 3. **p < .05 (Student’s t-test). (C,D) We detectec the isolation of nuclear and cytoplasmic RNA, circCDYL was mainly localized in the cytoplasm in both EJ and T24T cell lines. GAPDH and U1 were used as a negative control, respectively.
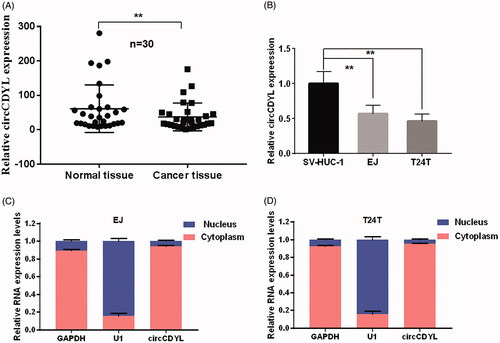
Table 1. Clinicopathological features of 30 bladder cancer patients and the expression of circCDYL.
Figure 3. Over-expression of circCDYL inhibits cell growth in bladder cancer cell lines. (A) The expression levels of circCDYL in EJ and T24T cells after stable transfection of circCDYL or vector plasmids were detected by quantitative real-time PCR. Data are mean ± SEM, n = 3. **p < .05(Student’s t-test). (B,C) Stable transfection of circCDYL in EJ and T24T cellssignificantly decrease the proliferation. Data are mean ± SEM, n = 3. **p < .05, ***p < .01 (Student’s t-test). (D) Flow cytometry showed that circCDYL induced cell cycle arrest at G0–G1 phase in both EJ and T24T cells compared with negative control.
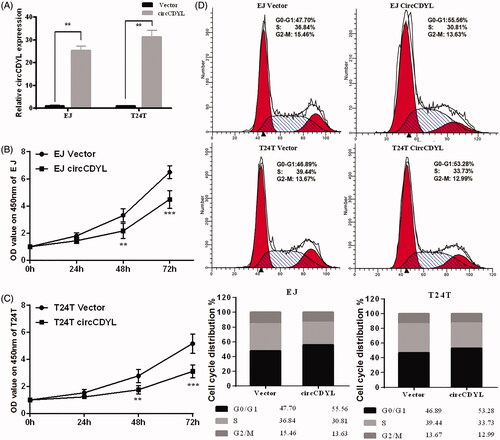
Figure 4. Over-expression of circCDYL inhibits cell migration in bladder cancer cell lines. (A) In wound healing assay, the migration of EJ and T24T cells transfected with circCDYL were significantly decreased. Data are mean ± SEM, n = 3. **p < .05 (Student’s t-test). Scale bar: 200 μm. (B) Transwell migration assays demonstrated that circCDYL decreased the invasion ability of EJ and T24T cells. Data are mean ± SEM, n = 3. **p < .05 (Student’s t-test). Scale bar: 100 μm.
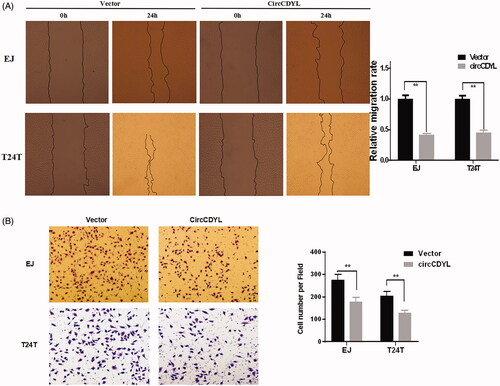
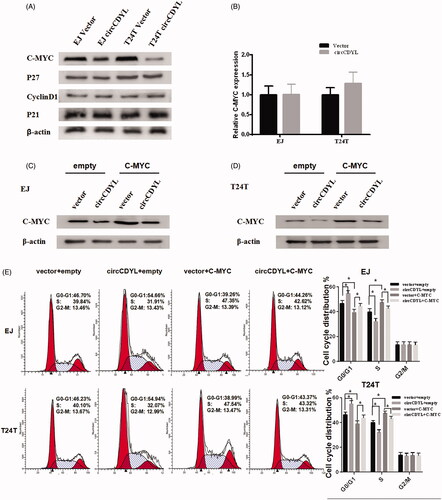
Figure 5. CircCDYL inhibits cell growth by down-regulating the protein level of C-MYC in bladder cancer. (A) Western blot indicating that over-expression of circCDYL down-regulated the expression of C-MYC in EJ and T24T cells, while the expression of P27, P21 and CyclinD1 were not changed. (B) Quantitative real-time PCR indicating that the mRNA levels of C-MYC were not changed by circCDYL transfected both in EJ and T24T. (C,D) Western blot indicating that the expression of C-MYC was significantly increased in the bladder cancer cells co-transfected with circCDYL plasmids and C-MYC, compared with the cells transfected with circCDYL alone. (E) The cell-cycle assay showed that compared with empty vector group, over-expression of C-MYC could promote cell-cycle from G0–G1 phase to S phase, and co-transfected with C-MYC and circCDYL could partly reversed the G0/G1 phase cell cycle arrest induced by circCDYL in both EJ and T24T cells. Data are mean ± SEM, *p < .05 (Student’s t-test).