Figures & data
Figure 1. Impact of AP on cell proliferation and apoptosis in HT22 with LPS administration. (A) HT22 cells were disposed with the diverse concentrations of AP (0, 20, 40, 80 and 100 μg/mL), cell viability measured through CCK-8; HT22 cells were preconditioned with 80 μg/mL AP and then disposed with LPS (1 μg/mL), (B) cell viability and (C and D) p53 and p21 were assessed through CCK-8 and western blot; (E) cell apoptosis and (F) apoptosis-associated factors were determined through flow cytometry and western blot . *p < .05, **p < .01, ***p < .001 vs Control; #p < .05, ###p < .001 vs LPS group.
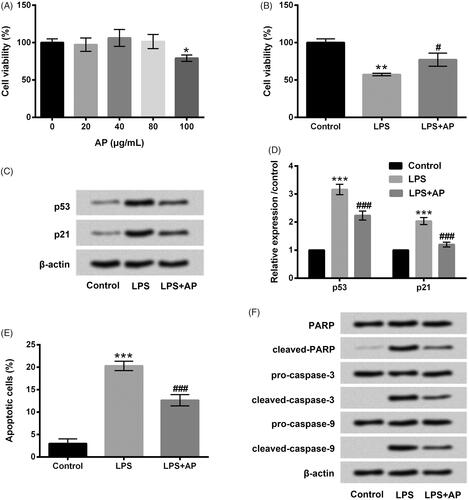
Figure 2. Impact of AP on the productions of pro-inflammatory cytokines in HT22 cells with LPS administration. HT22 cells were preconditioned with 80 μg/mL AP prior to dispose with LPS (1 μg/mL), (A) the mRNA levels and (B) concentrations of IL-1β, TNF-α and IL-6 were tested through RT-qPCR and ELISA. ***p < .001 vs Control; ###p < .001 vs LPS group.
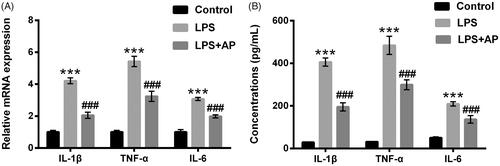
Figure 3. Impact of AP on miR-10a expression in HT22 cells with LPS stimulation. HT22 cells were preconditioned with 80 μg/mL AP prior to administrate with LPS (1 μg/mL) and miR-10a expression was determined through RT-qPCR. *p < .05 vs Control; ###p < 0.001 vs LPS group.
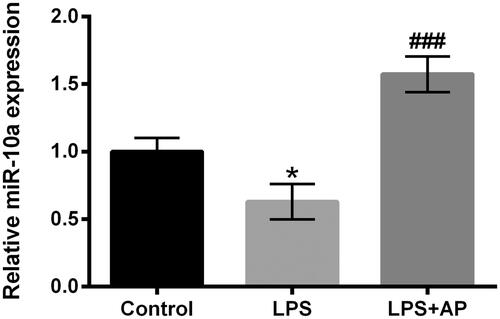
Figure 4. Impact of miR-10a inhibition on cell proliferation and apoptosis in HT22 cells co-stimulated with LPS and AP. HT22 cells were respectively transfected with miR-10a inhibitor and NC, (A) miR-10a expression in these cells tested through RT-qPCR; these cells were preconditioned with 80 μg/mL AP prior to dispose with LPS (1 μg/mL), (B) cell viability (C and D) p53 and p21 were gauged through CCK-8 and western blot; (E) cell apoptosis and (F) apoptosis-associated factors were assessed through flow cytometry and western blot. ***p < .001 vs Control; ###p < .001 vs LPS group; &&&p < .001 vs LPS + AP + NC.
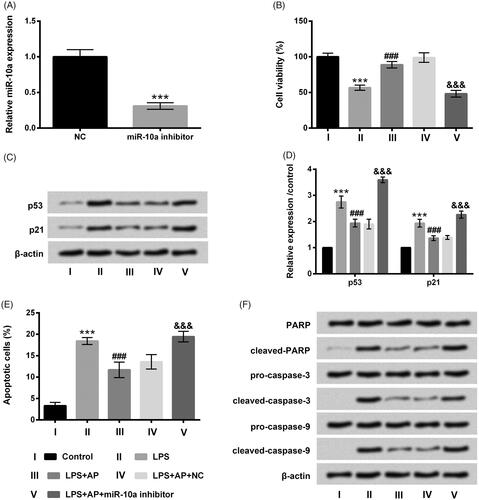
Figure 5. Impact of miR-10a inhibition on the productions of pro-inflammatory cytokines in HT22 cells co-stimulated with LPS and AP. HT22 cells were respectively transfected with miR-10a inhibitor and NC, and then were preconditioned with 80 μg/mL AP prior to dispose with LPS (1 μg/mL), (A) the mRNA levels and (B) the concentrations of IL-1β, TNF-α and IL-6 were assessed through RT-qPCR and ELISA. ***p < .001 vs Control; #p < .05, ###p < .001 vs LPS group; &&&p < .001 vs LPS + AP + NC.
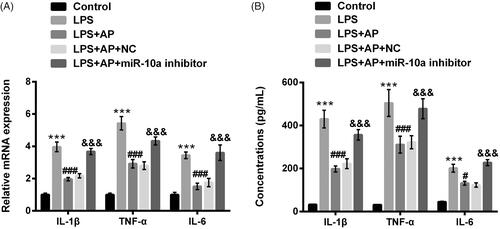
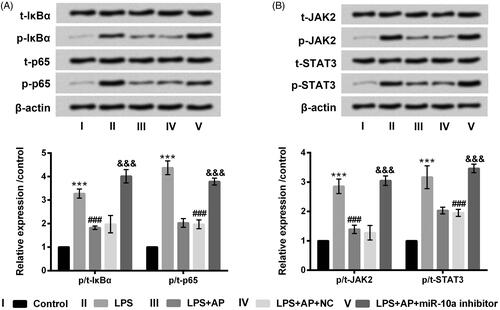
Figure 6. Impact of AP and miR-10a on NF-κB and JAK2/STAT3 pathways in HT22 cells with LPS treatment. HT22 cells were respectively transfected with miR-10a inhibitor and NC, and then were preconditioned with 80 μg/mL prior to dispose with LPS (1 μg/mL), (A) NF-κB pathway associated factors and (B) JAK2/STAT3 pathway associated factors examined through western blot. ***p < .001 vs Control; ###p < .001 vs LPS group; &&&p < .001 vs LPS + AP + NC.