Figures & data
Figure 1. LPS promoted inflammatory insults in ATDC5 cells. (A) ATDC5 cells were stimulated with LPS at the indicated concentrations (0, 1, 2, 5, and 10 μg/mL) for 12 h. The cell viability was analyzed with a CCK-8 assay. ATDC5 cells were treated with 5 μg/mL LPS for 12 h in the downstream experiments. (B) Apoptotic cells were stained with Annexin-FITC/PI and then observed with a flow cytometry. (C) Immunoblot assay was conducted to evaluate the expression of apoptosis-related proteins. (D) Inflammatory factors were quantified by qRT-PCR assay at mRNA level. (E) Immunoblot assay was performed to detect inflammatory factors. (F) ELISA was performed to assess the protein expression of inflammatory factors. Data was the mean of three independent experiments, each conducted in triplicate, ±SD. nsp > .05; *p < .05; **p < .01; ***p < .001. ns: not significant; LPS: lipopolysaccharide; CTRL: control; PARP: poly(ADP-ribose) polymerase; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha; CCK-8: cell counting kit-8; FITC/PI: fluorescein isothiocyanate/propidium iodide; qRT-PCR: quantitative reverse transcription PCR; ELISA: enzyme-linked immuno sorbent assay.
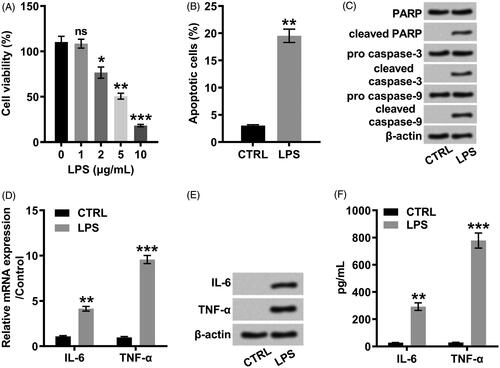
Figure 2. LPS positively modulated the expression of LINC00305 in a concentration-dependent relationship. LINC00305 was quantified by qRT-PCR. ATDC5 cells were treated by LPS at the indicated concentrations (0, 1, 2, and 5 μg/mL) for 12 h. Data was the mean of three independent experiments, each conducted in triplicate, ±SD. nsp > .05; *p < .05; **p < .01. ns: not significant; LPS: lipopolysaccharide; qRT-PCR: quantitative reverse transcription PCR.
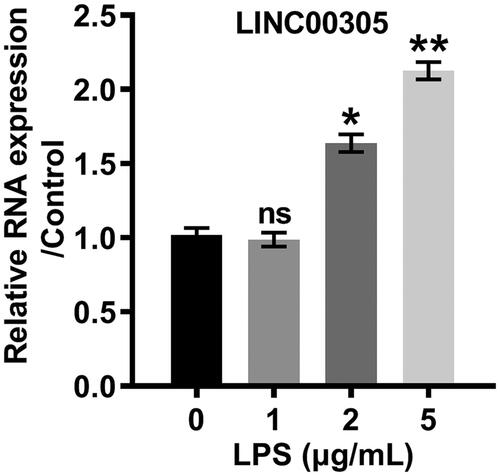
Figure 3. LINC00305 overexpression was necessary for LPS-mediated inflammatory damages in ATDC5 cells. (A) ATDC5 cells were transfected with LINC00305 or sh-LINC00305. The expression of LINC00305 was analyzed by qRT-PCR. ATDC5 cells were transfected and then treated with 5 μg/mL LPS for 12 h. (B) After transfection and LPS administration, ATDC5 cells viability was examined by CCK-8 assay. (C) Apoptotic cells were observed by a flow cytometry after stained by Annexin V-FITC/PI. (D) Immunoblot assay was used to determine apoptosis-related proteins. (E) Inflammatory factors at mRNA level were examined by qRT-PCR. (F) Immunoblot assay was performed to detect inflammatory factors. (G) Inflammatory factors at protein level were quantified by ELISA. Data was the mean of three independent experiments, each conducted in triplicate, ±SD. nsp > .05; *p < .05; **p < .01; ***p < .001. sh: short hairpin; CTRL: control; LPS: lipopolysaccharide; NC: negative control; PARP: poly(ADP-ribose) polymerase; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha; qRT-PCR: quantitative reverse transcription PCR; CCK-8: cell counting kit-8; FITC/PI: fluorescein isothiocyanate/propidium iodide; ELISA: enzyme-linked immuno sorbent assay.
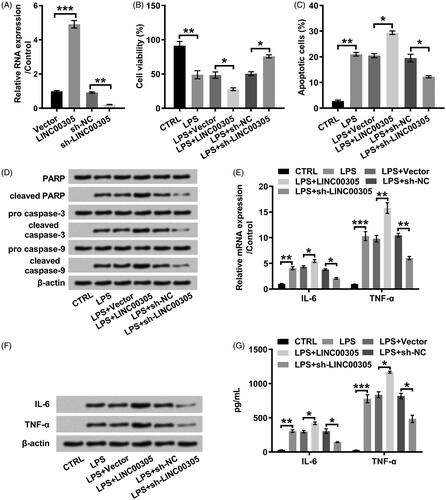
Figure 4. miR-124 was positively modulated by LINC00305. ATDC5 cells were transfected with LINC00305 or sh-LINC00305. miR-124 expression was evaluated by qRT-PCR. Data was the mean of three independent experiments, each conducted in triplicate, ±SD. *p < .05. qRT-PCR: quantitative reverse transcription PCR; miR-124: microRNA-124.
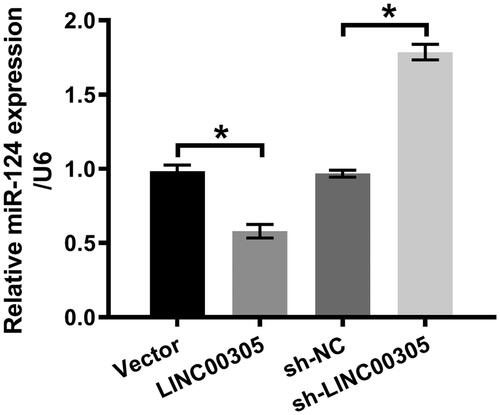
Figure 5. miR-124 down-regulation was required for LINC00305 to involve in inflammatory damages induced by LPS. (A) miR-124 mimic was transfected into ATDC5 cells and qRT-PCR was applied to examine miR-124 level. ATDC5 cells were transfected with LINC00305 and miR-124 mimic or NC and then treated with 5 μg/mL LPS for 12 h. (B) Cell viability and (C) apoptosis was assessed by CCK-8 and Annexin V-FITC/PI combined with a flow cytometry, respectively. (D) Immunoblot assay was conducted for quantification of proteins associated with apoptosis. The expression of inflammatory factors was evaluated by qRT-PCR, Western blot and ELISA at (E) mRNA and (F, G) protein levels. Data was the mean of three independent experiments, each conducted in triplicate, ±SD. *p < .05; **p < .01; ***p < .001. NC: negative control; miR-124: microRNA-124; CTRL: control; LPS: lipopolysaccharide; PARP: poly(ADP-ribose) polymerase; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha; qRT-PCR: quantitative reverse transcription PCR; CCK-8: cell counting kit-8; FITC/PI: fluorescein isothiocyanate/propidium iodide; ELISA: enzyme-linked immuno sorbent assay.
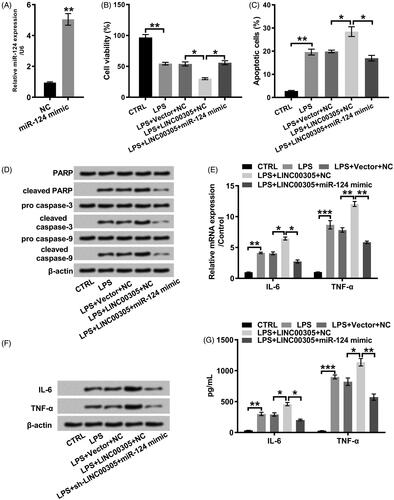
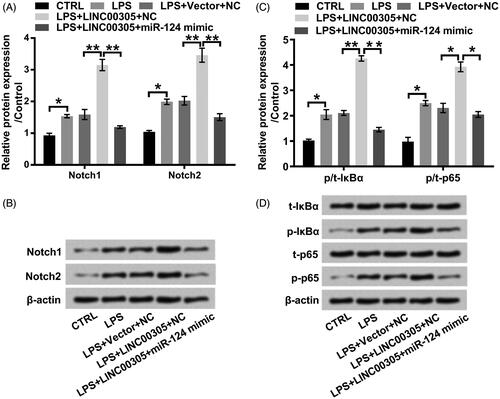
Figure 6. LINC00305-triggered activation of Notch/NF-κB pathway is abolished by miR-124 overexpression in the presence of LPS. ATDC5 cells were transfected with LINC00305 and miR-124 mimic or NC and then treated with 5 μg/mL LPS for 12 h. Immunoblot assay was performed to detect (A, B) Notch1, Notch2, (C, D) t-IκBα, p-IκBα, t-p65, and p-p65. Data was the mean of three independent experiments, each conducted in triplicate, ±SD. *p < .05; **p < .01. NC: negative control; miR-124: microRNA-124; CTRL: control; LPS: lipopolysaccharide; t: total; p: phospho; IκBα: inhibitor of nuclear factor kappa-B alpha.