Figures & data
Figure 1. Characterization of FITC-VHP-Fe3O4@SiO2. (a) The morphology of Fe3O4@SiO2 with different magnification under TEM (left: the scale bar is 0.2 μm; right: the scale bar is 50 nm). (b) FITR analyse of SiO2 (black line), FITC (green line), VHPKQHR (blue line) and FITC-VHP-Fe3O4@SiO2 (red line).
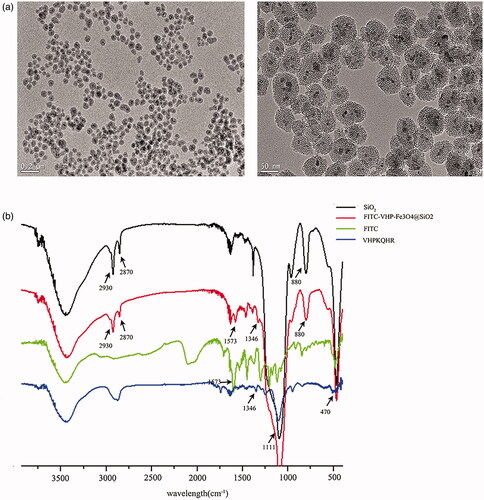
Figure 2. The binding ability of FITC-VHP-Fe3O4@SiO2 to endothelial cells with high expression of VCAM-1. (a) FITC-Fe3O4@SiO2, FITC-ACTH-Fe3O4@SiO2 and FITC-VHP-Fe3O4@SiO2 were incubated with stimulated MAECs and observed under confocal microscope. (b) The FITC fluorescence intensity corresponding to confocal microscope was calculated using Image J software. (c) The fluorescence intensity of MAECs after incubation with FITC-VHP-Fe3O4@SiO2 for 0, 0.5, 1, 2 h was analyzed by flow cytometry. ***p < .001.
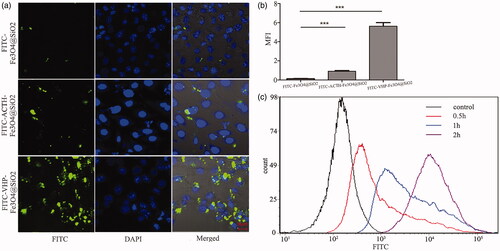
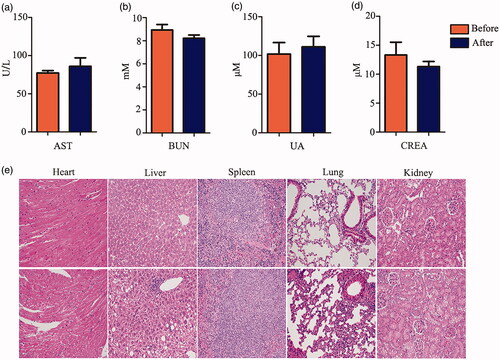
Figure 3. Biosafety of FITC-VHP-Fe3O4@SiO2 in vitro. (a) The hemolysis rate under FITC-VHP-Fe3O4@SiO2 for 10, 25, 50, 100, 200 μg/ml. (b) CCK8 was used to assess the effect of 10, 25, 50, 100, 200 μg/ml FITC-VHP-Fe3O4@SiO2 on viability of MAECs and Raw264.7. *p < .05; **p < .01; ##p < .01; ###p < .001.
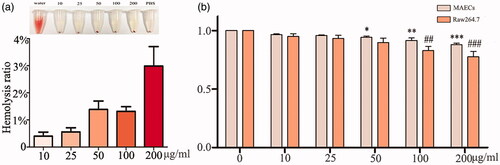
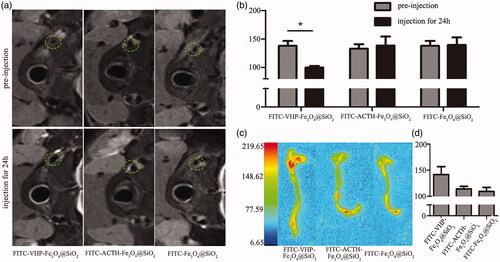
Figure 4. FITC-VHP-Fe3O4@SiO2 can target atherosclerotic plaques ex vivo and in vivo. T2 signal intensity (a) and T2 value (b) before and after treatment with FITC-Fe3O4@SiO2, FITC-ACTH-Fe3O4@SiO2 and FITC-VHP-Fe3O4@SiO2 for 24 h. The fluorescence intensity distribution of blood vessels (c) and Quantitative analysis of fluorescence intensity (d) was measured using a Small Animal Multi-mode Imaging System after injecting with FITC-Fe3O4@SiO2, FITC-ACTH-Fe3O4@SiO2 and FITC-VHP-Fe3O4@SiO2 for 24 h. * p < .05