Figures & data
Figure 1. Characterization of biosynthesized Siberian ginseng gold nanoparticle. The UV–visible spectrum absorption pattern at different period of time (A) and selected area diffraction pattern (B) of SG-GNPs.
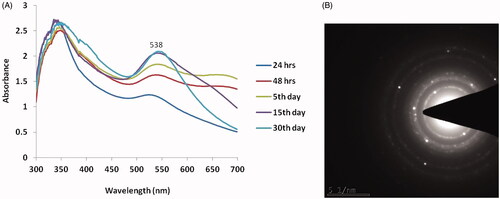
Figure 2. Characterization of biosynthesized Siberian ginseng gold nanoparticle. High Resolution Transmission electron microscopy (TEM) (A) and X ray diffraction analysis (XRD) of SG-GNPs (B).
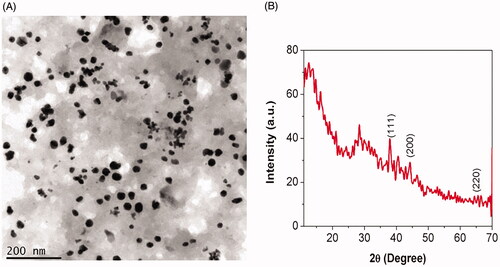
Figure 3. Characterization of biosynthesized Siberian ginseng gold nanoparticle. Fourier-transform infrared spectroscopy analysis of SG-GNPs.
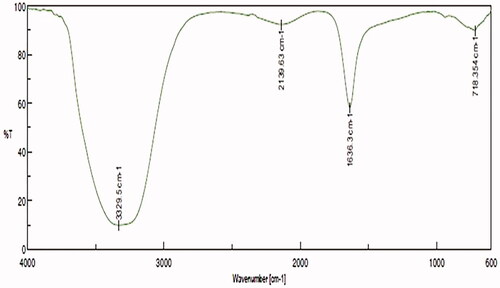
Figure 4. Anticancer activity of biosynthesized Siberian ginseng gold nanoparticle. The CC50 dose of SG-GNPs against murine melanoma B16 cells were assessed by MTT assay. B16 cells were treated with different concentration of SG-GNPs ranging from 0.5 to 20 µg/ml and incubated for 24 h. The control and SG-GNPs treated cells were subjected to MTT assay and the results were statistically analyzed. Each bar represents mean ± SEM of three independent observations. p < .05 is considered as statistically significant.
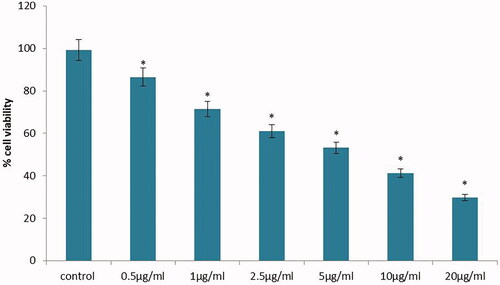
Figure 5. Anticancer activity of biosynthesized Siberian ginseng gold nanoparticle. Apoptotic effect of SG-GNPs on mitochondrial membrane premeability in murine melanoma cell line B16 was assessed using 1 mM Rhodamine 123 staining technique. B16 murine melanoma cells were treated with 5 and 10 µg/ml SG-GNPs and incubated for 24 h. The control and treated cells were then stained with Rhodamine 123 stain for 15 min. The experiments were performed in triplicates.
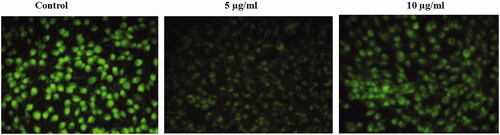
Figure 6. Anticancer activity of biosynthesized Siberian ginseng gold nanoparticle. Apoptotic effect of SG-GNPs in murine melanoma cell line B16 was assessed using dual staining technique. B16 murine melanoma cells were treated with 5 and 10 µg/ml SG-GNPs and incubated for 24 h. The control and treated cells were then stained with 1 mM acridine orange and 1 mM Ethidium bromide for 5 min. The experiments were performed in triplicates.
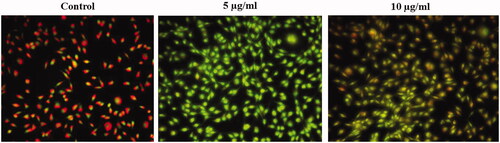
Figure 7. Anticancer activity of biosynthesized Siberian ginseng gold nanoparticle. Apoptotic effect of SG-GNPs via generation of reactive oxygen generation in murine melanoma cell line B16 was assessed using H2DCFDA staining technique. B16 murine melanoma cells were treated with 5 and 10 µg/ml SG-GNPs and incubated for 24 h. The control and treated cells were then stained with 2',7'-dichlorodihydrofluoresceindiacetate stain for 15 min. The experiments were performed in triplicates.
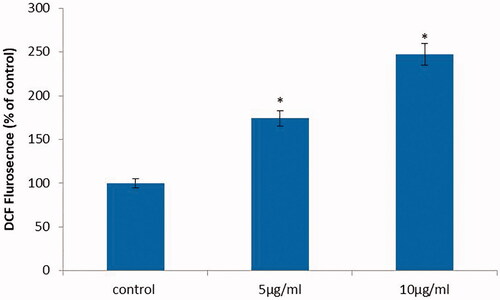
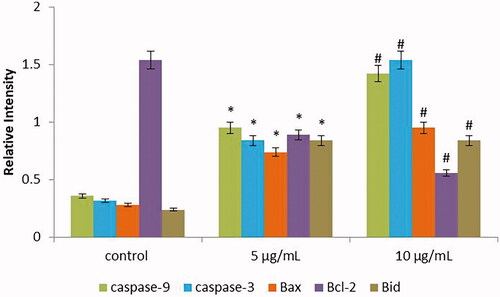
Figure 8. Anticancer activity of biosynthesized Siberian ginseng gold nanoparticle. Apoptotic effect of SG-GNPs on apoptotic gene expression in B16 murine melanoma cells was assessed using qPCR analysis. B16 murine melanoma cells were treated with 5 and 10 µg/ml SG-GNPs and incubated for 24 h. After incubation, the control and treated cells were subjected to total RNA isolation. 2 µg of total RNA from control and SG-GNPs treated group were converted to cDNA and subjected to qPCR analysis with specific apoptotic genes Caspase 3, Caspase 9, Bid, Bax and antiapoptotic gene Bcl2. The experiments were performed in triplicates.