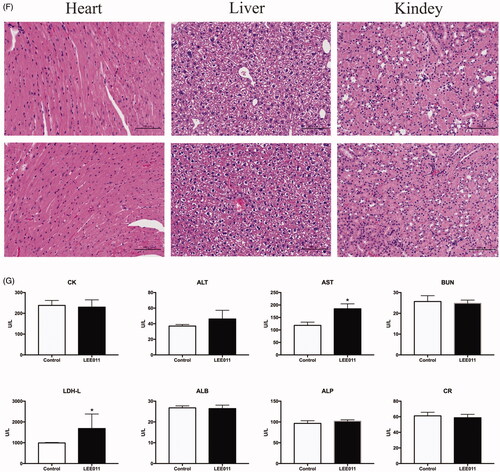
Figures & data
Figure 2. LEE011 suppressed cell proliferation and promoted apoptosis by inhibiting the CDK4/6-cyclin D-Rb-E2F pathway in MDA-MB-231. CDK4, CDK6, cyclin D1 levels were detected by Western blotting after treatment with LEE011 for 72 hours and the expression of proteins which connected to CDK4/6-cyclin D-Rb-E2F axis, as well as apoptosis, was simultaneously assessed.
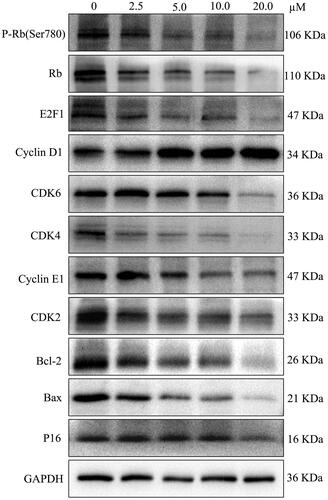
Figure 3. LEE011 induced cell cycle G1 phase arrest and promoted cell apoptosis in MDA-MB-231. After culturing the MDA-MB-231 cells with increasing concentrations of LEE011 for 72 hours, we applied FACS to evaluate cell proliferation and cell apoptosis. (A–C) Representative images of cell cycle distribution in MDA-MB-231 were displayed after exposure to LEE011 for 72 hours. When treated with 2.5 µM of LEE011, G0/G1 portion increased with 11.58%, S portion decreased with 9.4%, and G2/M portion decreased with 1.13%. In 5.0 µM of LEE011 incubated group, G0/G1 portion increased with 17.96%, S portion decreased with 12.72%, and G2/M portion decreased with 5.72%. Besides, G0/G1 portion increased with 26.24%, S and G2/M portion decreased with 16.53%, 9.56%, respectively in 10.0 µM group compared to the control. Cell numbers of different cell cycle phases in different treatment groups were counted and analyzed statistically. Two-tailed Student’s t-test was utilized to determine statistical significance. Error bars indicate standard deviations. p < .05 was considered significant compared with the 0µM group (*p < .05, **p < .01, ***p < .001). (D,E) Representative images of cell apoptosis in MDA-MB-231 were displayed after 72 hours-LEE011 exposure. Alive cells are shown in the lower left part of the panel (Q4); early apoptotic cells are shown in the lower right part of the panel (Q3); late apoptotic cells are shown in the higher right part of the panel (Q2); necrotic cells are shown in the higher left part of the panel(Q1). LEE011 (2.5, 5.0, 10.0 µM) led to 1.5 times (14.93%), 1.6 times (16.17%), and 2.2 times (21.75%) of apoptosis separately than control (9.95%). Cell apoptosis of different treatment groups were counted and analyzed statistically. Two-tailed Student’s t-test was performed to determine statistical significance. Error bars indicate standard deviations. p < .05 was considered significant compared with the 0 µM group (**p < .01, ***p < .001).
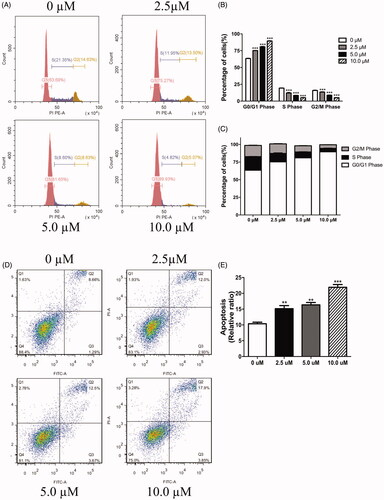
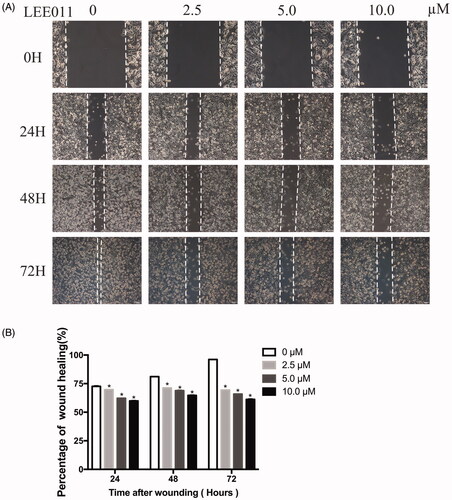
Figure 4. LEE011 reduces cell migration of MDA-MB-231 in vitro. Cell migration was determined by wound healing assays after an escalating dose (of LEE011 treatment imposed on MDA-MB-231 cells for the indicated time. (A) Representative images of MDA-MB-231 cell migration after 0, 2.5, 5.0, 10.0 µM LEE011 exposure for 24, 48, 72 hours. The magnification is 200× (scale bar, 100 µm). (B) Cell migration of MDA-MB-231 was measured after LEE011 exposure. Two-tailed Student’s t-test was utilized to determine statistical significance. Error bars indicate standard deviations. p < .05 was considered significant (*p < .05).