Figures & data
Figure 1. RSC96 cells were injured by high glucose (HG). RSC96 cells were cultivated in HG condition for 48 h. Cells in normal glucose (NG) were considered as control. (A) Cell viability, (B) apoptosis rate, (C,D) cleavage of caspase-3 and PARP, (E) ROS generation, and (F,G) expression of neurotrophic proteins were examined by CCK-8 assay, flow cytometry, Western blot and DCFH-DA probe. Data were presented as mean ± SD (n = 3). *p < .05 (Student’s t-test).
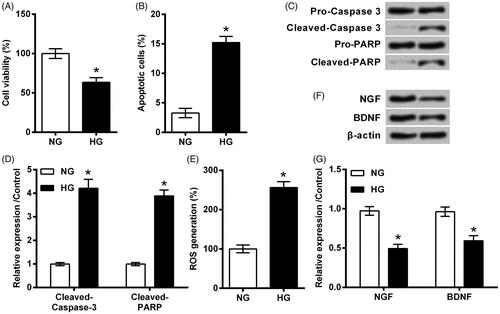
Figure 2. Notoginsenoside R1 (NGR1) pre-conditioning ameliorated high glucose (HG)-evoked injury in RSC96 cells. (A) NGR1 with various dosages ranged from 10 to 100 μM was utilized to treat RSC96 cells. (B) RSC96 cells were pre-treated with NGR1 and then stimulated by HG. Cell viability was examined by CCK-8 kit. 50 μM was applied as optimum dosage for use in following experiments. (C) Apoptosis rate, (D,E) cleavage of caspase-3 and PARP, (F) ROS generation and (G,H) expression of neurotrophic proteins were examined by flow cytometry, Western blot and DCFH-DA probe. Data were presented as mean ± SD (n = 3). *p < .05 (ANOVA).
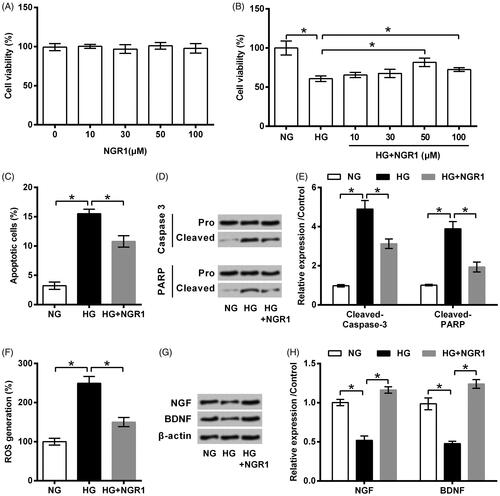
Figure 3. Notoginsenoside R1 (NGR1) prevented high glucose (HG)-induced miR-503 expression. RSC96 cells were pre-treated with NGR1 and then stimulated by HG. Expression of miR-503 was examined by qRT-PCR. Data were presented as mean ± SD (n = 3). *p < .05 (ANOVA).
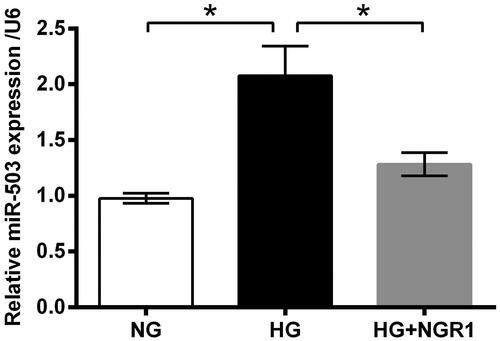
Figure 4. Notoginsenoside R1 (NGR1) pre-conditioning ameliorated high glucose (HG)-evoked injury in RSC96 cells through miR-503. (A) miR-503 mimic and its respective control (NC mimic) were transfected into RSC96 cells. Transfection efficiency was examined by qRT-PCR. The transfected or non-transfected cells were then treated by NGR1, HG or both. (B) Cell viability, (C) apoptosis rate, (D,E) cleavage of caspase-3 and PARP, (F) ROS generation, and (G,H) expression of neurotrophic proteins were examined by CCK-8 assay, flow cytometry, Western blot and DCFH-DA probe. Data were presented as mean ± SD (n = 3). *p < .05 (ANOVA).
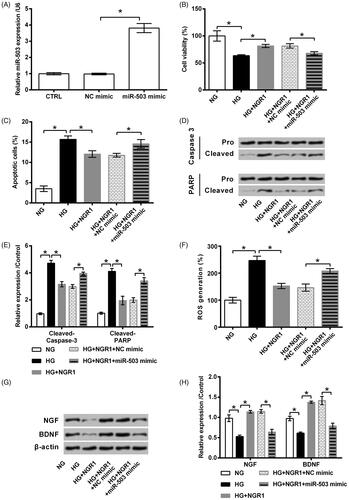
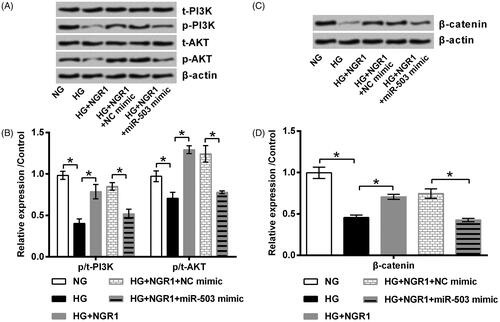
Figure 5. Notoginsenoside R1 (NGR1) activated PI3K/AKT and β-catenin signalling through miR-503. miR-503 mimic and its respective control (NC mimic) were transfected into RSC96 cells. The transfected or non-transfected cells were then treated by NGR1, high glucose (HG) or both. Expression of (A, B) PI3K, AKT and (C,D) β-catenin was examined by Western blot. Data was presented as mean ± SD (n = 3). *p < .05 (ANOVA).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
