Figures & data
Figure 1. Tumour cells, including LL/2, CT26 and B16F10, were transfected with an hFAPα expression vector (line 2) and an empty vector pVector (line 1) via the Lipofectamine 3000 reagent, and wild-type tumour cells (line 3) were used as controls. (A) hFAPα expression levels in murine tumour cells analyzed by a Western blot. (B) The DPP activity levels of hFAPα produced by tumour cells. Data are expressed as the means ± SDs (n = 3), and one-way ANOVA was used to analyze the statistical significance. *p < .05. The DPP activity levels of hFAPα produced by tumour cells transfected with hFAPα expression vector compared to controls.
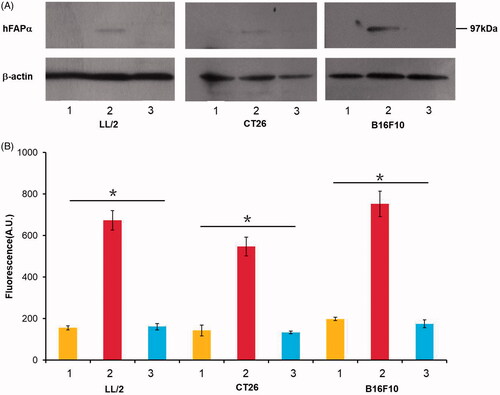
Figure 2. Vaccination with a hFAPα-expressing WCTV restrains the tumour growth of tumour-bearing mice. (A) After transplantation of 1 × 106 tumour cells into the right flank on day 0, mice were vaccinated with 1 × 106 inactivated hFAP-transduced, pVector-transduced cancer cells or wild-type cancer cells through subcutaneous injection (s.c.) on the bilateral axillaries and the unilateral inguinals on days 5, 12 and 19. Normal saline (n.s.) was used as a negative control. Each experimental group was tested twice. (B–D) The tumour volume in mice immunized with an hFAPα-expressing WCTV or controls in the three tumour models. Values are presented as the means ± SDs (n = 10). The statistical significance was analyzed using one-way ANOVA. **p < .01; ***p < .001 tumour volume of hFAP-LL/2, hFAP-CT26, hFAP-B16F10 differs from control groups. (E–G) Survival of mice after different vaccinations (n = 10). The log-rank (Mantel–Cox) test was utilized to calculate statistical significance. *p < .05; ***p < .001 survival of hFAP-LL/2, hFAP-CT26, hFAP-B16F10 compared to control groups.
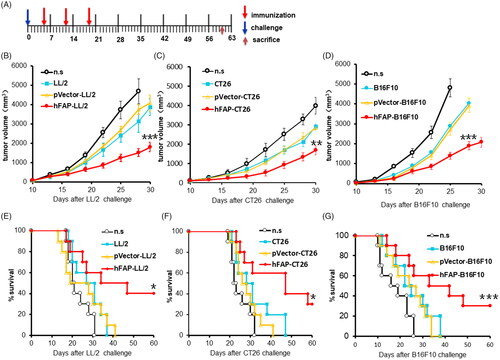
Figure 3. The anti-tumour effect of hFAP-LL/2 involved cellular immune responses. (A, B) Ability of the adoptive transfer of splenocytes from syngeneic mice to protect recipient mice from tumour growth in the LL/2 model. Splenocytes (1 × 107 cells per mouse) collected from different treatment groups were injected into the tail veins of the recipient mice. Significant differences in tumour volume of the mice that received spleen cells from hFAPα-expressing WCTV-treated mice differ from controls are presented by asterisks (***p < .001), and they exhibited a markedly increased survival rate compared to controls (***p < .001). (C) The revocation of the anti-tumour effect by the exhaustion of immunocyte subsets. The anti-tumour effect induced by hFAPα-expressing WCTVs was completely abrogated by the exhaustion of CD8+ T cells. Depletion of CD4(+) T cells partly affected the abrogation of anti-tumour activity, while the depletion of NK cells did not obstruct the development of anti-tumour immunity. (D) CTL-mediated cytotoxicity in vitro by the 51Cr release assay. T lymphocytes isolated from hFAPα-expressing WCTV-treated mice showed increased cytotoxicity to CAFs and LL/2 tumour cells (p < .05). (E) CD8 (FITC) and CD4 (PE) antibodies were used to stain single cell suspensions of the lymph nodes of nearby primary tumours harvested from immunized mice, and a remarkably increased percentage of CD8+ T cells was found in mice immunized with hFAP-LL/2.
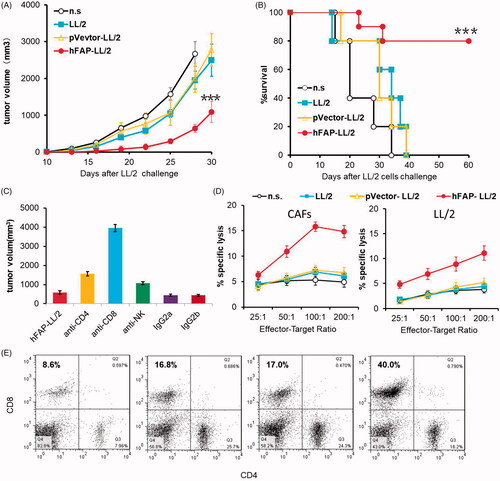
Figure 4. LL/2 tumour sections were harvested from C57BL/6J mice that were immunized once a week for three weeks after the tumour cell injection. (A, B) H&E staining and the TUNEL assay of tumour tissues. Tumour sections from mice treated with hFAP-LL/2 showed more lymphocytes and apoptotic cancer cells than those in the control groups. Scale bar, 100 µm. (C, D) Expression of FAPα and α-SMA within tumours from mice immunized with hFAP-LL/2, pVector-LL/2, LL/2 and normal saline. Scale bar, 100 µm.
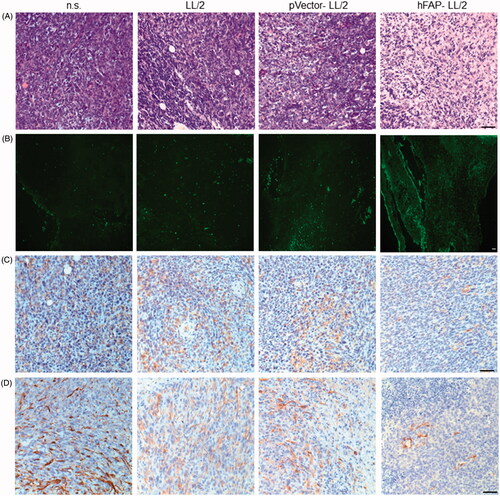
Figure 5. Protective anti-tumour responses. (A) C57 mice (n = 10) were vaccinated with irradiated hFAP-transduced, pVector-transduced, wild-type tumour cells or n.s. on D-21, D-14 and D-7. Mice were then injected subcutaneously with 1 × 106 tumour cells on D0. (B, C) There was a notable difference in tumour volume among vaccinated mice and those in the control groups. ***p < .001 tumour volume of hFAP-LL/2, hFAP-B16F10 differs from control groups. (D, E) Significant reduction in tumour-free rate. **p < .01; ***p < .001 tumour free rate of hFAP-LL/2, hFAP-B16F10 compared to controls.
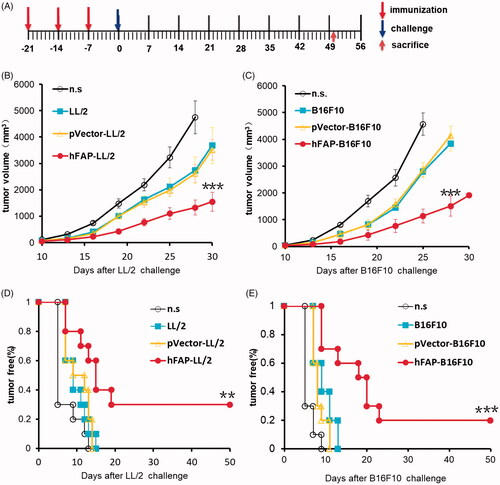
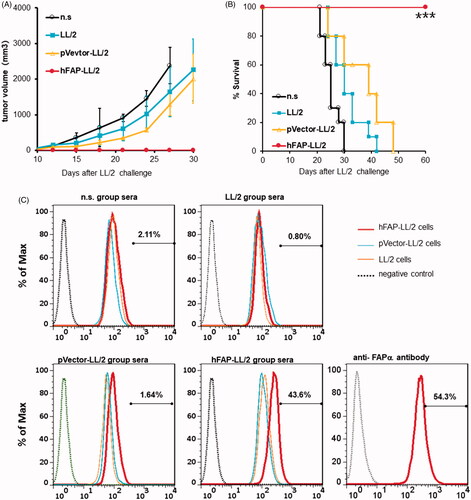
Figure 6. Anti-tumour activity of the hFAPα-expressing WCTV involved in humoural immune responses. (A, B) Ability of adoptive transfer of immunoglobulins from syngeneic mice to protect recipient mice from tumour growth in the LL/2 model. Purified immunoglobulins (300 µg/mg) from mice vaccinated with different vaccines were injected into the tail veins of recipient animals. Inhibition of subcutaneous tumour growth and apparently increased survival (***p < .001) was found when mice received immunoglobulins from hFAPα-expressing WCTV-treated mice. (C) Exponentially growing LL/2, pVector-LL/2 and hFAP-LL/2 tumour cells were incubated with purified immunoglobulins in mice from different vaccine groups. The polyclonal rabbit anti-FAPα antibody incubation cells were used as a positive control. Autoantibodies against FAPα were detected in the sera of hFAPα-expressing WCTV-immunized mice via flow cytometry.