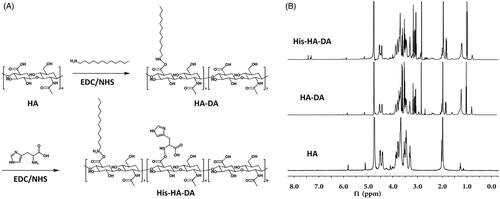
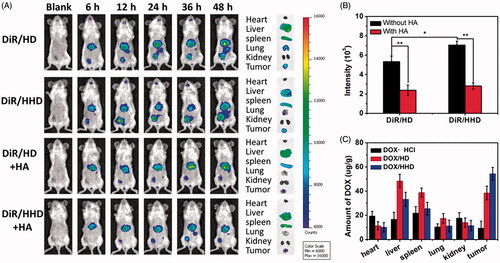
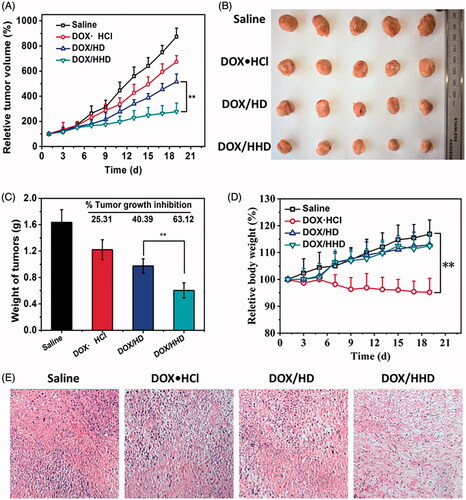
Figures & data
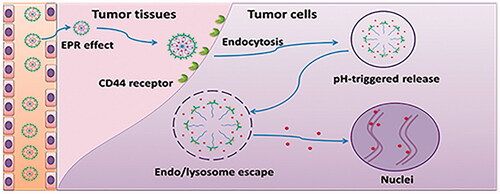
Scheme 1. CD44 targeted pH-responsive micelles for the delivery of DOX in vivo. (A) Self-assembly and pH-responsive behaviour of the micelles. (B) Delivery of DOX with the CD44 targeted pH-responsive micelles.
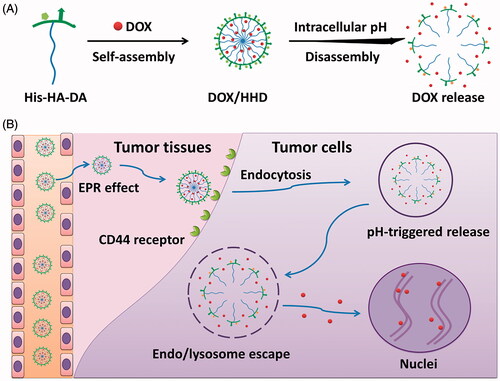
Table 1. Characteristic of DOX-loaded micelles.
Table 2. IC50 of DOX in different formulations against 4T1 cells.
Figure 2. pH-responsive study of DOX/HHD. (A). CMC of HHD at pH 7.4 or 5.3; (B). Particle size of DOX/HD and DOX/HHD; (C). Particle size of DOX/HHD at pH 7.4 or 5.3; (D). Zeta potential of DOX/HHD at pH 7.4 or 5.3; (E). EE% of DOX/HHD at pH 7.4 or 5.3; (F). Release of DOX from DOX.HCl, DOX/HD or DOX/HHD at pH 7.4 or 5.3.
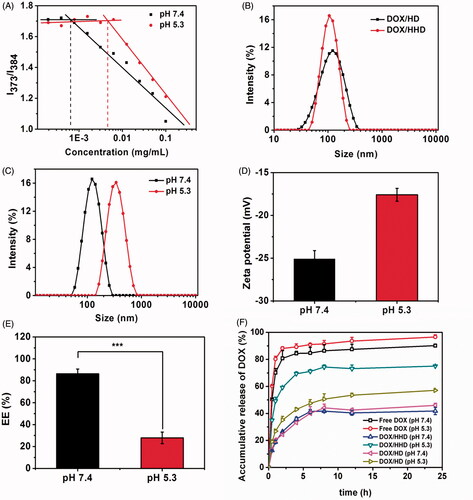
Figure 3. Cellular uptake of DOX in different formulations. (A) Cellular uptake of DOX-loaded micelles with or without free HA CLSM (40×). (B) Cellular uptake of DOX in different formulations via flow cytometry. (10 μg/mL DOX concentration).
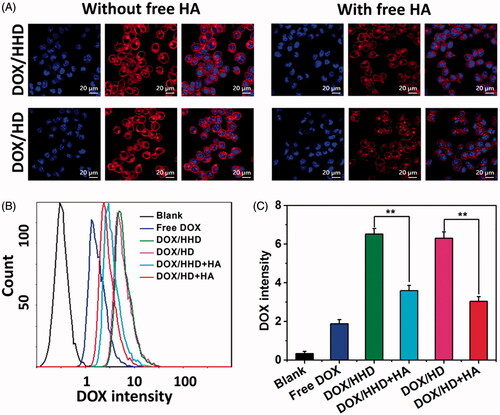
Figure 4. Intracellular trafficking of DOX-loaded micelles. (A) Intracellular distribution of DOX/HHD or DOX/HD (10 μg/mL DOX concentration) (63×). (B) AO staining of cells treated with blank HHD or HD micelles (20×).
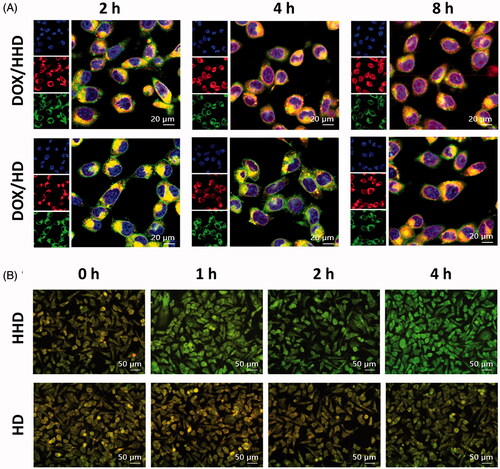
Figure 5. Cytotoxicity of DOX in different formulations against 4T1 cells (A. 24 h, B. 48 h, n = 5).
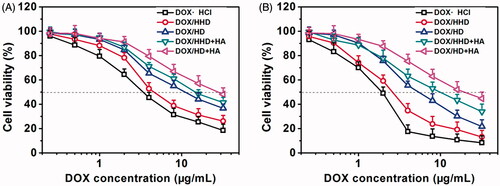
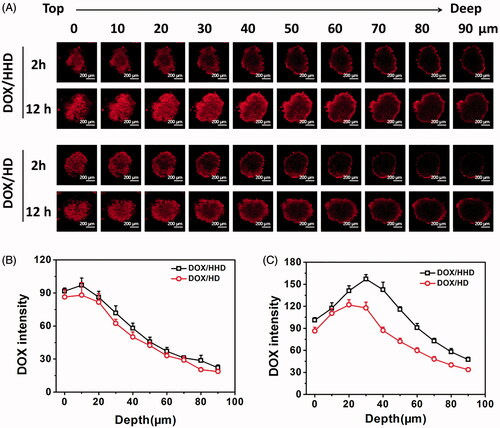
Figure 6. DOX distribution in 3 D tumour spheres after incubation for 2 or 12 h (A) (10×); DOX intensity in tumour spheres after incubation for 2 (B) or 12 h (C).