Figures & data
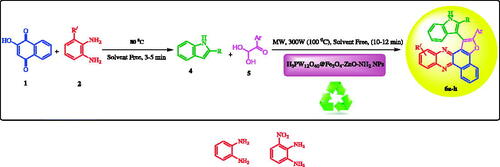
Scheme 1. Synthesis of benzo[a]furo[2, 3-c]phenazine derivatives in the presence of H3PW12O40@Fe3O4-ZnO as heterogeneous catalyst.
![Scheme 1. Synthesis of benzo[a]furo[2, 3-c]phenazine derivatives in the presence of H3PW12O40@Fe3O4-ZnO as heterogeneous catalyst.](/cms/asset/97275c34-c005-45e1-98f2-e208c47190f7/ianb_a_1894163_sch0001_c.jpg)
Table 1. Optimization of reaction conditions of compound 6a.
Table 2. Sequential one-pot four-component synthesis of benzo[a]furo[2, 3-c]phenazine derivatives.
Table 3. Comparison of results obtained using H3PW12O40@Fe3O4–ZnO with results obtained using other catalyst reported in the literature for the synthesis.
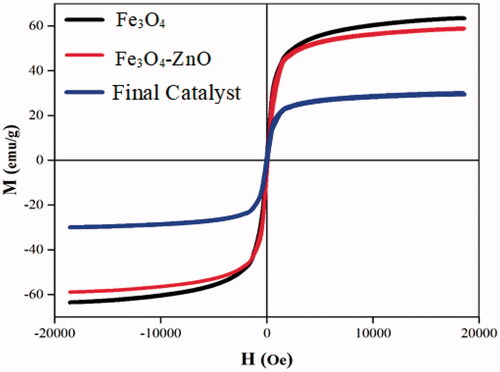
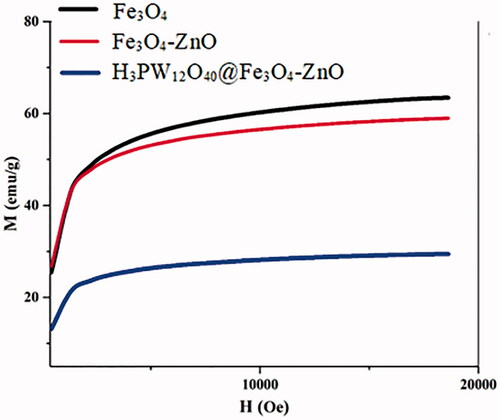
Table 4. Coercion field values, saturation magnetization and residual magnetization.


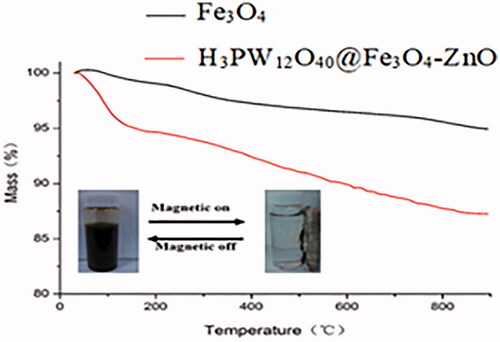
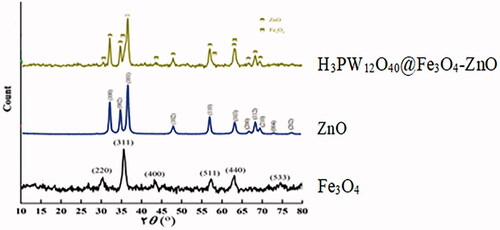
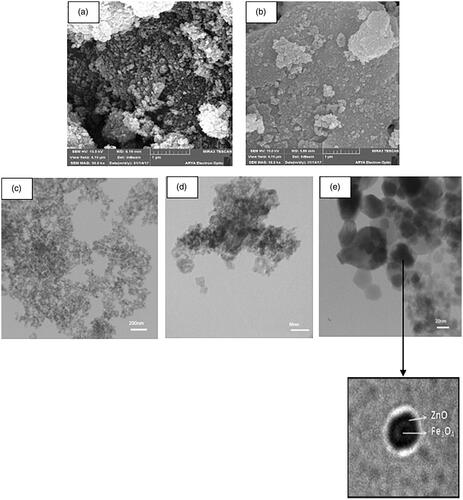
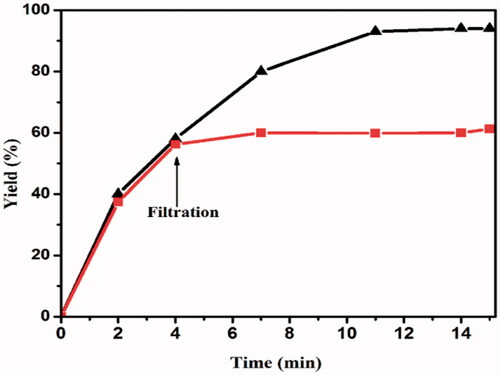
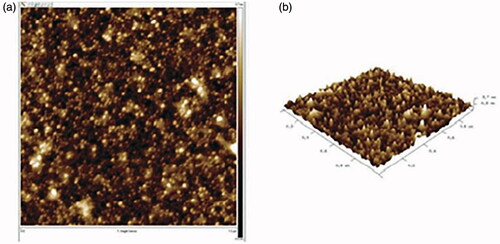
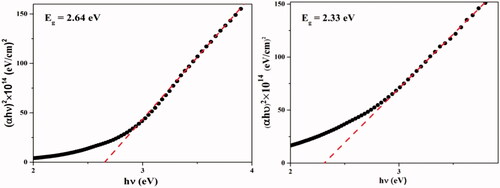
![Figure 13. Proposed mechanism for the synthesis of benzo[a]furo[2, 3-c]phenazine derivatives.](/cms/asset/1a5f56f3-28c4-407d-81f4-ac5d8e782871/ianb_a_1894163_f0013_c.jpg)