Figures & data
Figure 1. (a) Methods of producing droplets by extrusion dripping. (b) Microfluidic flow focusing device for droplet generation. This figure was drawn by the authors.
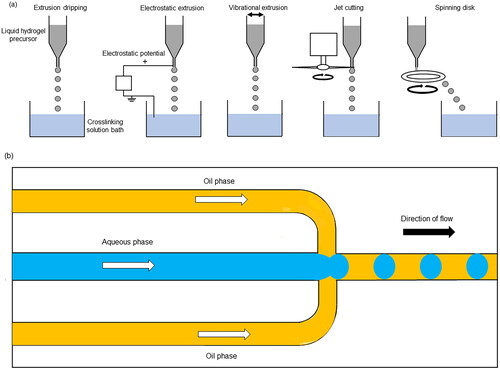
Figure 2. Simple pipetting method to create multilayered collagen-alginate beads. (a) First layer of alginate formed by pipetting through a hole in the substrate. (b) Second layer of collagen and cells (red) added. (c) Third layer of collagen (green) to form a double-layered droplet. (d) Small amount of alginate added to the top of the droplet. (e) Resulting multilayered hydrogel beads. Figure taken from [Citation45] and reproduced with permission from Springer Nature.
![Figure 2. Simple pipetting method to create multilayered collagen-alginate beads. (a) First layer of alginate formed by pipetting through a hole in the substrate. (b) Second layer of collagen and cells (red) added. (c) Third layer of collagen (green) to form a double-layered droplet. (d) Small amount of alginate added to the top of the droplet. (e) Resulting multilayered hydrogel beads. Figure taken from [Citation45] and reproduced with permission from Springer Nature.](/cms/asset/f338f7c0-3953-4aff-bae3-713a1937c2e3/ianb_a_2359996_f0002_c.jpg)
Figure 3. Production of microencapsulated prostate cancer bone metastasis niche model via pipetting onto a superhydrophobic surface. (a) Schematic production of microencapsulated co-culture bone metastasis niche model using methacrylated gelatin (GelMA) and methacrylated hyaluronan (HA-MA). (b) Widefield fluorescence micrographs of cell-specific tracking in microencapsulated models. Prostate cancer cells (PC-3) were stained with DiO (blue), and human osteoblasts (hOB) with DiD (red). Scale bars = 200 µm. Figure taken from [Citation46] and reproduced with permission from Elsevier.
![Figure 3. Production of microencapsulated prostate cancer bone metastasis niche model via pipetting onto a superhydrophobic surface. (a) Schematic production of microencapsulated co-culture bone metastasis niche model using methacrylated gelatin (GelMA) and methacrylated hyaluronan (HA-MA). (b) Widefield fluorescence micrographs of cell-specific tracking in microencapsulated models. Prostate cancer cells (PC-3) were stained with DiO (blue), and human osteoblasts (hOB) with DiD (red). Scale bars = 200 µm. Figure taken from [Citation46] and reproduced with permission from Elsevier.](/cms/asset/2b6f73e1-5c27-4a1d-b2a0-54b37997e666/ianb_a_2359996_f0003_c.jpg)
Figure 4. Microfluidic droplet generation for a breast cancer cell and fibroblast co-culture microencapsulation model. (a) Droplet generation process using alginate (Alg) or alginate-alginate sulphate (Alg/Alg-S). (b) Resulting droplets with encapsulated MCF7 cells labelled with CFSE (green) and CCD1129SK human mammary fibroblasts labelled with CMAC (blue). Upper panel: 5X magnification, after droplet formation; lower panel: 20X maginification, after hydrogel crosslinking. Scale bar in (a) and (b) upper panels = 200 µm. Scale bar in (b) lower panels = 50 µm. Figure taken from [Citation58] reproduced with permission from Elsevier.
![Figure 4. Microfluidic droplet generation for a breast cancer cell and fibroblast co-culture microencapsulation model. (a) Droplet generation process using alginate (Alg) or alginate-alginate sulphate (Alg/Alg-S). (b) Resulting droplets with encapsulated MCF7 cells labelled with CFSE (green) and CCD1129SK human mammary fibroblasts labelled with CMAC (blue). Upper panel: 5X magnification, after droplet formation; lower panel: 20X maginification, after hydrogel crosslinking. Scale bar in (a) and (b) upper panels = 200 µm. Scale bar in (b) lower panels = 50 µm. Figure taken from [Citation58] reproduced with permission from Elsevier.](/cms/asset/dab9e7aa-8965-4148-881b-023170808be9/ianb_a_2359996_f0004_c.jpg)
Figure 5. Core-shell gelatin-alginate microparticles for cervical cancer cell encapsulation produced using a hybrid extrusion and microfluidic approach. (a–d) HeLa cell growth and spheroid formation after encapsulation in two-layered microcapsules. (e) Spheroids harvested from microcapsules after 18 days of cultivation. (f) Spheroids formed on agarose gel after 9 days of cultivation. Scale bar in (a–d) = 150 μm; scale bar in (f) = 300 μm. Figure taken from [Citation63] reproduced with permission from John Wiley and Sons.
![Figure 5. Core-shell gelatin-alginate microparticles for cervical cancer cell encapsulation produced using a hybrid extrusion and microfluidic approach. (a–d) HeLa cell growth and spheroid formation after encapsulation in two-layered microcapsules. (e) Spheroids harvested from microcapsules after 18 days of cultivation. (f) Spheroids formed on agarose gel after 9 days of cultivation. Scale bar in (a–d) = 150 μm; scale bar in (f) = 300 μm. Figure taken from [Citation63] reproduced with permission from John Wiley and Sons.](/cms/asset/68d66798-0d1a-4406-bc48-6183aca24730/ianb_a_2359996_f0005_c.jpg)
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
