Figures & data
Table 1. The demographic information and clinical symptoms of recruited COVID-19 patients.
Figure 1. Virus positive rates in throat swab (TS) and anal swab (AS) samples. (A) The time course of positive rates in TS and AS samples on each day after symptom onset is shown (left part). The average viral RNA positive rates in TS and AS samples shown in the column were compared (right part). (B) The weekly positive rates in TS and AS samples after symptom onset. (C) The virus positive rates in TS and AS samples in survivors and non-survivors. (D) The weekly positive rates in survivors and non-survivors.
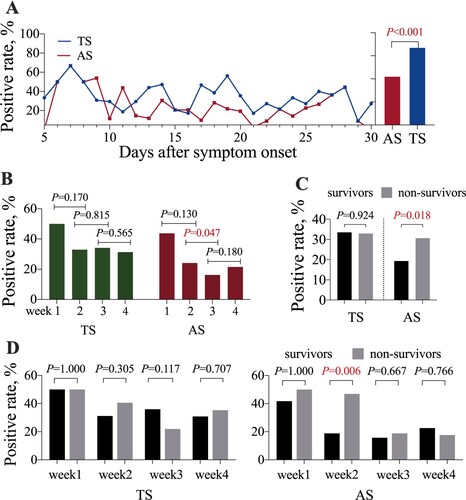
Figure 2. The time to SARS-CoV-2 positivity in throat swab (TS) and anal swab (AS) samples. (A) The time from symptom onset to viral RNA positivity in TS and AS samples. (B) The time from symptom onset to viral RNA positivity in TS and AS samples from survivors and non-survivors. (C) The time to viral RNA negativity after a positive result in TS and AS samples. (D) The time to viral RNA negativity after a positive result in TS and AS samples from survivors and non-survivors.
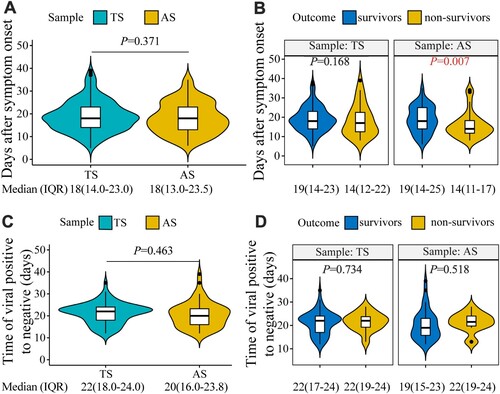
Figure 3. Viral load dynamics in throat swab (TS) and anal swab (AS) samples from patients with COVID-19. (A) The viral load in serial samples collected every 4–7 days. (B) The weekly mean viral load in the specimens post symptom onset. (C) The viral loads in specimens from survivors and non-survivors. (D) The weekly mean viral loads in specimens from patients with different outcomes post symptom onset.
Table 2. Association between initial viral load and adverse outcomes of COVID-19 patients.
