Figures & data
Figure 1. Design of linker-immunodominant site (LIS) COVID-19 vaccine candidates. (a) Structural representation and (b) schematic view of the immunodominant sites in the SARS-CoV-2 spike protein. Abbreviations: SP, signal peptide; NTD, N-terminal domain; RBD, receptor-binding domain; FP, fusion peptide; HR, heptad repeat; TM, transmembrane domain; CP, cytoplasmic domain. (c) Schematic depiction of the RBD-ID and S-ID gene sequences. (d) The amino acid sequences of the 10 immunodominant sites. (e) Representation of the predicted RBD-ID and S-ID structures. (f) SDS-PAGE analysis of the recombinant RBD-ID (14 kDa) and S-ID (48 kDa).
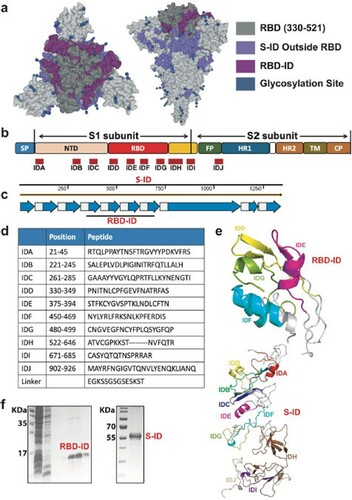
Figure 2. Humoral immune responses in RBD-ID-iummunized or S-IDimmunized BALB/c mice. The BALB/c mice were immunized subcutaneously with 25 μg recombinant RBD-ID (n = 6), S-ID (n = 6), or aluminum hydroxide gel (control, n = 6) per mouse, and boosted with an equivalent dose 21 days later. Serum samples were collected from the mice at days 7, 14, 21, and 28 after the initial immunization and tested for binding antibody and neutralizing antibody titers. (a) Schedule of immunization and sample collection. (b) IgG subclass binding antibodies against RBD-ID or RBD in mice immunized with RBD-ID detected by ELISA. Data are presented as mean ± SD. Statistical significance was calculated using a two-way ANOVA with multiple comparisons tests (*p < 0.05, **p < 0.01, ***p < 0.001). (c) IgG subclass binding antibodies against S-ID or RBD in mice immunized with SID detected by ELISA. Data are presented as mean ± SD. Statistical significance was calculated using a two-way ANOVA with multiple comparisons tests (*p < 0.05, **p < 0.01, ***p < 0.001). (d) Endpoint titer ratios of IgG2a/c to IgG1. Data are shown as mean ± SD. (e) % neutralization and (f) 50% neutralizing antibody titer achieved by RBD-ID, S-ID, or control in the sera of immunized mice. Neutralization was determined using VSVbased SARS-CoV-2 pseudovirus. The dashed line indicated the detection limit or and the solid line indicated 50% neutralization. Data are shown as mean ± SD.
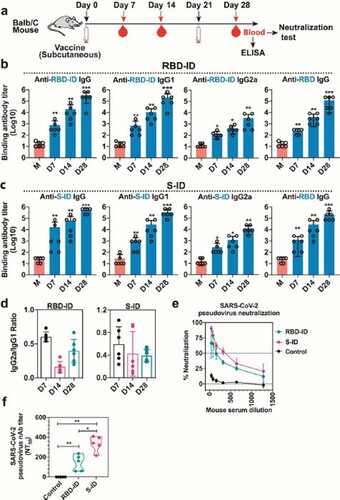
Figure 3. Humoral immune responses in immunized golden Syrian hamsters. The hamsters were immunized subcutaneously with 25 μg recombinant RBD-ID (n = 5), SID (n = 5), or aluminum hydroxide gel (control, n = 5) per hamster, and boosted with an equivalent dose 21 days later. Serum samples were collected from the mice at days 7, 21, and 28 after the initial immunization and tested for binding antibody and neutralizing antibody titers. (a) Schedule of immunization and sample collection. (b) IgG subclass binding antibodies against RBD-ID, S-ID, or RBD in hamsters immunized with RBD-ID (left) or S-ID (right) detected by ELISA. Data are presented as mean ± SD. Statistical significance was calculated using a two-way ANOVA with multiple comparisons tests (*p < 0.05, **p < 0.01, ***p < 0.001). (c) Immunodominant site-specific antibodies in the hamsters. (d) % neutralization and (e) 50% neutralizing antibody titer achieved by RBD-ID, SID, or control in the sera of immunized hamsters. Neutralization was determined using VSV-based SARS-CoV-2 pseudovirus. The dashed line indicated the detection limit or and the solid line indicated 50% neutralization. Data are shown as mean ± SD.
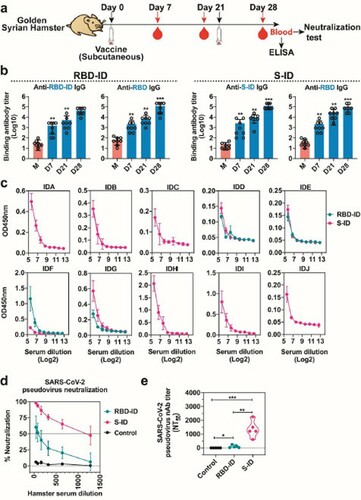
Figure 4. Cellular immune responses in RBD-ID-iummunized or S-ID-immunized BALB/c mice. The BALB/c mice were immunized subcutaneously with 25 μg recombinant RBD-ID (n = 6), S-ID (n = 6), or aluminum hydroxide gel (control, n = 6) per mouse, and boosted with an equivalent dose 21 days later. Splenocytes were collected at days 28 after the initial immunization and tested for T cell response. (a) Schedule of immunization and sample collection. (b) ELISPOT assay for IFN-γ and IL-4 in the splenocytes of the mice immunized with RBD-ID or (c) S-ID. Data are shown as violin plot with all points. Unpaired two-tailed Student's t-test, **p < 0.01. Abbreviations: SI, immunized mice stimulated with RBDID or S-ID; SM, mock-immunized mice stimulated with RBD-ID or S-ID.
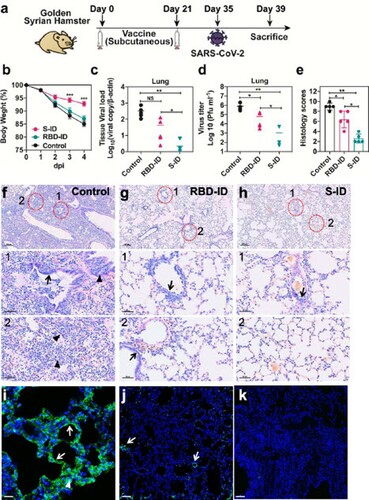
Figure 5. S-ID immunization protects golden Syrian hamsters from severe SARSCoV-2 infection. At 35 days after the initial immunization, hamsters (n = 5 per group) were intranasally inoculated with 105 plaque forming units (pfu) of SARS-CoV-2 in 100 µl DMEM. The hamsters were sacrificed at 4 days after virus challenge for tissue collection for viral loads, virus titer, and lung pathology evaluation. (a) Schedule of immunization, virus challenge, and tissue collection. (b) Body weight changes of the hamsters. Error bars denote SD. (c) The viral loads and (d) virus titers in the lungs of the hamsters at 4 days after virus challenge determined by quantitative RT-PCR and median tissue culture infective dose (TCID50) assay, respectively. Data are presented as mean ± SD. Statistical significance was calculated using a two-way ANOVA with multiple comparisons tests (*p < 0.05, **p < 0.01, ***p < 0.001). (e) Histological scores indicating the lung histopathological severity in each group. Data are presented as mean ± SD of five randomly selected slides from each group. Unpaired two-tailed Student's t-test, **p < 0.01 when compared with the placebo group. (f) to (h) Representative images of the H&E-stained lung tissues of (f) mockimmunized, (g) RBD-ID-immunized, or (h) S-ID-immunized hamsters at 4 days after SARS-CoV-2 challenge. The mock-immunized hamsters showed diffuse lung tissue consolidation with alveolar infiltration and exudation, bronchiolar epithelium cell death (arrows), and peribronchiolar mononuclear cell infiltration (arrowheads). These changes were less severe in the RBD-ID-immunized hamsters and the least severe in the S-ID-immunized hamsters. (i) to (k) Representative images of the immunofluorescent staining using SARS-CoV2 nucleocapsid protein of the lung tissues of (i) mock-immunized, (j) RBD-IDimmunized, or (k) S-ID-immunized hamsters at 4 days after SARS-CoV-2 challenge. The mock-immunized hamsters showed abundant SARS-CoV-2 nucleocapsid protein expression diffusely distributed in the alveola (white arrowheads) and in the bronchiolar epithelial cells (white arrows). Scale bar, 100 μm. The RBD-ID-immunized hamsters showed markedly less SARS-CoV-2 nucleocapsid protein expression and the S-IDimmunized hamsters exhibited almost no detectable SARS-CoV-2 nucleocapsid protein expression.
COVID19_LIS_Vaccine-_SM_2021_03_31_editable.docx
Download MS Word (5.7 MB)Data availability
All data is available upon request. Unique materials used in this study are available from the corresponding author by Material Transfer Agreement.
