Figures & data
Figure 1. Immunization schedule and immunobiological analysis using the antigens of monkeypox A29L protein and A35R protein. (A), Schedule of A29L protein and A35R protein immunization, serum collection, mAbs generation, and VACV challenge. (B), Homology analysis of monkeypox A29L protein and homologous antigens from other orthopoxviruses. (C), Analysis of sera binding activity from A29L protein immunized mice. (D), Analysis of sera binding activity from A35R protein immunized mice. (E), Neutralization titre analysis of sera from A29L protein and A35R protein immunized mice against IMV form of VACV (Tian tan) strain. (F), Neutralization titre analysis of sera from A29L and A35R immunized mice against IMV form of VACV (WR) strain. (G), Neutralization titre analysis of sera from A29L protein and A35R protein immunized mice against EEV form of VACV (Tian tan) strain. (H), Neutralization titre analysis of sera from A29L protein and A35R protein immunized mice against EEV form of VACV (WR) strain.
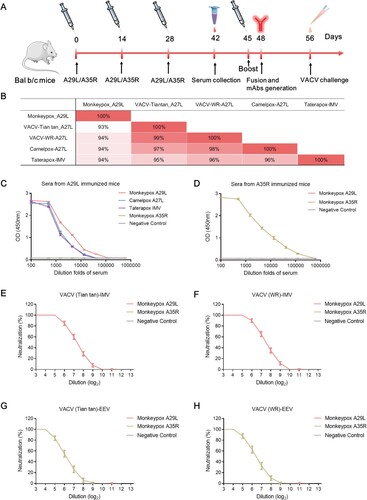
Figure 2. In vivo protection of mice immunized with monkeypox-specific A29L protein or A35R protein and number of mAbs generation from immunized mice. (A), Changes in body weight of mice immunized with monkeypox virus-specific A29L protein or A35R protein after being challenged with vaccinia virus Tiantan strain. (B), Number of monkeypox-specific monoclonal antibodies screened from A35R protein and A29L protein immunized mice. (C), CDR sequences of six A29L protein -specific monoclonal antibodies
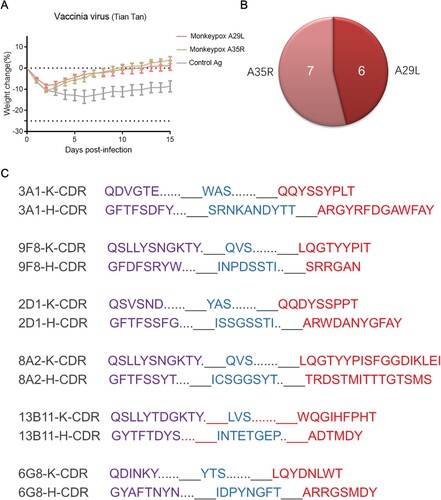
Figure 3. Analysis of ELISA and microneutralization activities of monkeypox A29L protein and A35R protein specific monoclonal antibodies. (A-D), ELISA activity of monkeypox A29L protein specific monoclonal antibodies against monkeypox A29L protein (A), Camelpox A27L protein (B), Taterapox A27L protein (C) and IMV form protein of VACV Tian tan strain (D). (E-F), ELISA activity of monkeypox A35R specific monoclonal antibodies against monkeypox A35R protein (E) and EEV form of VACV Tian tan strain (F). (G-H), microneutralization activity of monkeypox A29L protein specific monoclonal antibodies against IMV form of VACV Tian tan strain (G) and IMV form of VACA WR strain (H). (I), microneutralization activity of monkeypox A35R specific monoclonal antibodies against EEV form of VACV Tian tan strain.
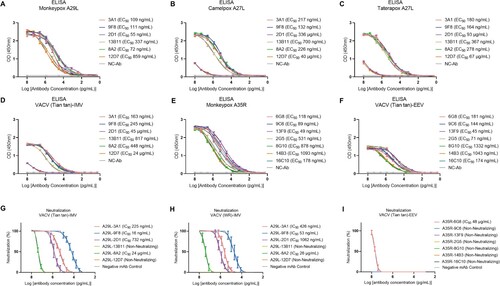
Figure 4. Epitope competition analysis and synergetic antiviral effects of monkeypox A29L protein specific antibodies 3A1,9F8 and 2D1. (A), 3A1 epitope comparison with 9F8 and 2D1 using competition ELISA,with 3A1 as competitor. (B), 9F8 epitope comparison with 3A1 and 2D1 using competition ELISA,with 2D1 as competitor. (C), 2D1 epitope comparison with 3A1 and 9F8 using competition ELISA, with 2D1 as competitor. (D), Negative antibody epitope comparison with 3A1, 9F8, and 2D1 using competition ELISA. IgG was used as the competitor and as a non-neutralizing monoclonal antibody specific for A35R generated in our laboratory. Statistical analysis was performed using the t-test. *p < 0.05, **p < 0.05, ***p < 0.001 versus the negative control group. (E-F), Synergetic microneutralization ability of monkeypox A29L protein specific antibodies 3A1,9F8 and 2D1 against IMV form of VACV Tian tan strain (E) and IMV form of VACA WR strain (F).
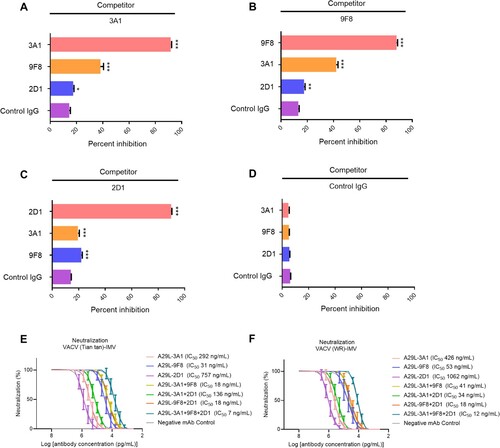
Figure 5. Model of A29L protein binding by 9F8, 2D1 and 3A1. The structures of A29L protein and the Fv domains of 9F8, 2D1 and 3A1 were predicted by AlphaFold2 and SabPred, respectively. The docked complexes were created by ZDOCK and ClusPro and were further investigated manually with epitope mapping information. The result that matches best with epitope mapping information was presented. (A-C), Overall architecture of A29L protein-9F8 (A), A29L protein-2D1 (B) and A29L protein-3A1 (C). The P1, P2 and P3 sections of A29L protein are coloured in magenta, violet and light pink, respectively. The 2D1 heavy chain, light chain and P4-P6 sections of A29L protein are coloured in blue, cyan and gray, respectively. The CDRs on both heavy chain and light chain are coloured in green. (D-F), The interfaces of A29L protein-9F8 (D), A29L protein-2D1 (E) and A29L protein-3A1 (F). The Fv domains of the antibodies are shown in surface representation with the heavy chain, light chain and CDRs coloured in blue, cyan and green, respectively. The residues on the heavy chain and light chain that are involved in A29L protein binding are labelled in white and orange. A29L protein is shown in cartoon and coloured in magenta. The residues involved in 9F8, 2D1 or 3A1 binding are shown in sticks. (G-I), The detailed interactions between A29L protein and CDRs on 9F8, 2D1 and 3A1. Residues on A29L protein are shown in stick representation and coloured in magenta. Residues on 9F8, 2D1 or 3A1 are shown in stick representation and coloured in green. The CDR domains which are unlikely to interact with A29L protein are not shown. Each residue is labelled by its one-letter code.
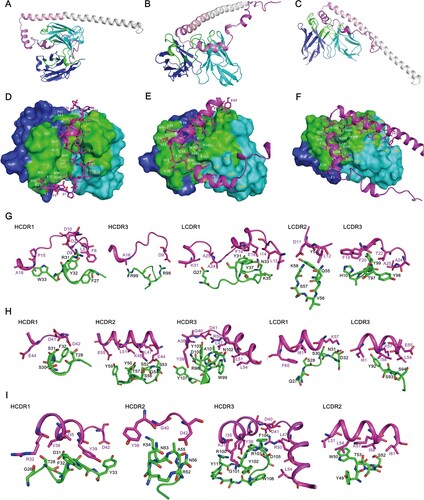
Figure 6. Prophylactic effects of monkeypox A29L protein specific antibodies 3A1,9F8, 2D1, 3A1 + 9F8, 3A1 + 2D1, 9F8 + 2D1, 3A1 + 9F8 + 2D1 in mice. A-B, Body weight changes of BALB/C mice (n = 6 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (A) or VACA WR strain (B) 24 h before intraperitoneal administration with the indicated antibodies (10 mg/kg). Weight curves represent the mean ± 95% confidence interval. c-d, Survival curves of BALB/C mice (n = 6 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (C) or VACA WR strain (D) 24 h before intraperitoneal administration with the indicated antibodies (10 mg/kg). E-F, Pulmonary virus titres of BALB/C mice (n = 5 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (E) or VACA WR strain (F) 24 h before intraperitoneal administration with the indicated antibodies (10 mg/kg). Black bars indicate mean values. For (E) and (F), the virus titres in the lungs of the mice treated with each antibody prophylactically were determined on four days after infection, the t-test was used for comparisons between groups. ***p < 0.001 versus the negative control group.
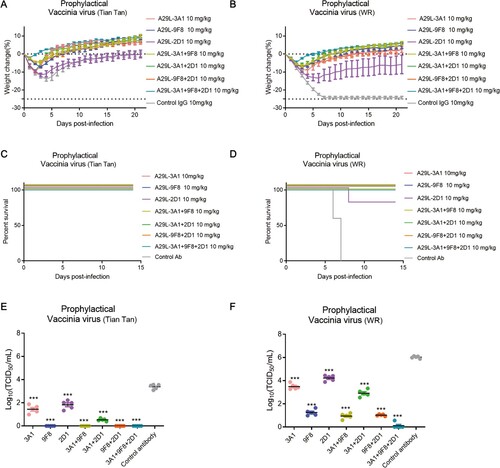
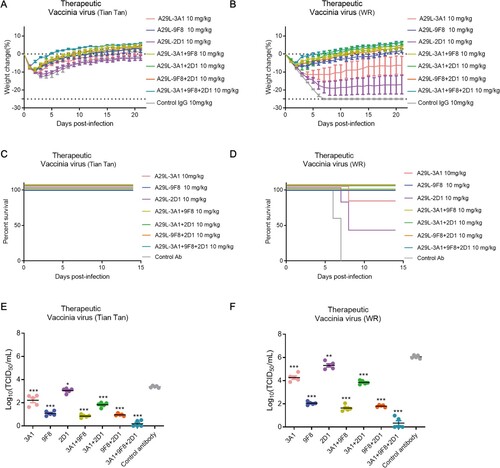
Figure 7. Therapeutic effects of monkeypox A29L protein specific antibodies 3A1,9F8, 2D1, 3A1 + 9F8, 3A1 + 2D1, 9F8 + 2D1, 3A1 + 9F8 + 2D1 in mice. A-B, Body weight changes of BALB/C mice (n = 6 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (A) or VACA WR strain (B) 24 h after intraperitoneal administration with antibodies (10 mg/kg). Weight curves represent mean ± 95% confidence interval. C-D, Survival curves of BALB/C mice (n = 6 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (C) or VACA WR strain (D) 24 h after intraperitoneal administration with antibodies (10 mg/kg). E-F, Pulmonary virus titres of BALB/C mice (n = 5 per group) infected with 5*106 TCID50 doses of the VACV Tian tan strain (E) or VACA WR strain (F) 24 h after intraperitoneal administration with antibodies (10 mg/kg). Black bars indicate mean values. For (E) and (F), virus titres in the lungs of mice treated with each antibody therapeutically were determined four days after infection; t-test was used for comparisons between groups. *p < 0.05, **p < 0.05, ***p < 0.001 versus the negative control group.
Supplemental Material
Download MS Word (3.4 MB)Data availability statement
The datasets generated and/or analyzed in the current study are available from the corresponding author upon reasonable request. The source data are provided in this study.
