Figures & data
Table 1. Summary of materials and methods for both studies showing the population, hardware, software, algorithms, and cross-validation procedures. Abbreviations: minimum distance to mean (MDM) is a Riemannian classifier; support vector machine (SVM) is a classifier. xDAWN is a spatial filter for event-related potentials.
Figure 1. The RoBIK prototype: the RoBIK headset (left) with 14 wet EEG channels and the virtual keyboard interface ‘Brainmium’ (right).
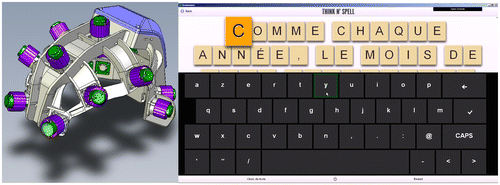
Figure 2. Patients were randomly assigned to try first the RoBIK BCI or the scanning device. The RoBIK prototype was evaluated within half a day while the scanning device was evaluated over three sessions in order to account for a possible learning effect. Four different texts of equivalent difficulty were selected so that the RoBIK device was evaluated with text 1 or 4.
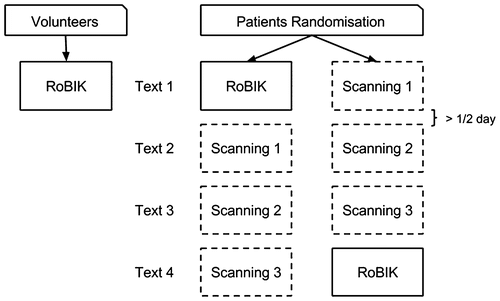
Table 2. Description of the population included in Study 1 with results: AC, access to computer; HM, voluntary head mobility; OE, oral expression; MV, mechanical ventilation, ET, endotracheal intubation. ‘Treatments’ details drugs administered to patients during the 48 h preceding the inclusion in the study. Training session performance is evaluated with a 20-fold cross-validation procedure on the training data using a combination of xDAWN and SVM. It shows discrimination – area under the receiver operating curve (AUROC) – between targets (33% of stimuli, i.e. One line and one column) and non-targets (random classifier is 50%). Testing and online performance shows accuracy in letter selection (1 symbol in 36; chance is 2.7%). The last lines of the table summarize the distributions: categorical variables are represented by the proportion of each category (noted in parenthesis) and continuous variables are represented by the mean and standard deviation; whenever relevant, the sample size is indicated in parenthesis. For speller performance, brackets indicate the average number of letters per patient. P-values are computed with a Mann-Whiteney U-test (*).
Table 3. Participants enrolled in Study 2 (patients and healthy volunteers). Abbreviations: ABP, arterial blood pressure – systolic (s) and diastolic (d); bpm, beats per minute; F, female; GBS, Guillain-barré syndrome; HR, heart rate; LIS, locked-in syndrome, M, male; mmHg, millimetres of mercury; MS, mulitple sclerosis; Q, quadriplegic; S, scanning device.
Figure 3. Comparison of setup time (min), self-evaluated comfort, spelling accuracy (%), and bit-rates (bit/min) between the RoBIK system (online results) and the three sessions of the scanning device.
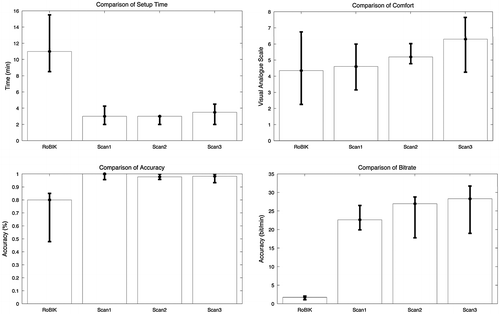
Figure 4. Comparison of the evolution of fatigue (left) and information rate (right) for patients (dark line) and healthy volunteers (bright line) over three times of the BCI protocol (train, online, free).
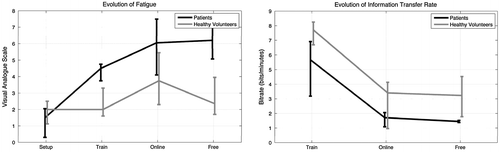
Figure 5. Example of two event-related potentials (ERPs) elicited from the interface used in the pilot study (left) and the RoBIK prototype (right); the RoBIK prototype does not show the typical steady-state visual evoked potential (SSVEP) in response to non-target stimulations that can be seen on the non-target response (left).
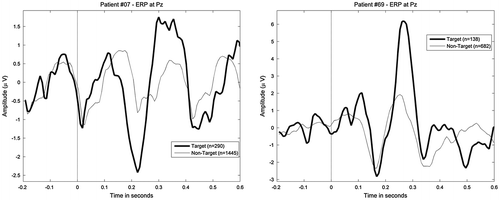
Table 4. Summary of materials and methods for both studies showing the population, hardware, software, algorithms, and cross-validation procedures used in each phase. Abbreviations: minimum distance to mean (MDM) is a Riemannian classifier; support vector machine (SVM) is a classifier; stepwise linear discriminant analysis (swLDA) is a linear classifier; xDAWN is a spatial filter for event-related potentials; P1, P2, and V2 refer to patients and volunteers included in clinical studies 1 and 2, respectively.
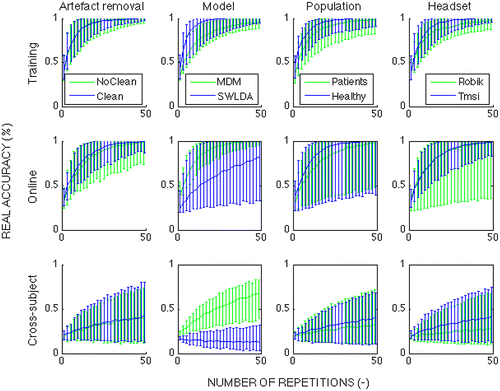
Figure 6. Evolution of ‘real accuracy’ (% of correctly identified characters) in relation to number of flashes of each symbol under different conditions; the three rows of plots indicate the performance in (1) cross-validated training data, (2) test-set (online) data, and (3) cross-subject performance. The first column of plots shows the effect of artifact removal using the SQI on the data from Study 1 only. The second column shows the impact of the classifier comparing xDAWN + stepwise linear discriminant analysis (SWLDA) and minimum distance to mean (MDM) Riemannian classifier. The third column compares the performance of healthy volunteers and patients. The fourth column compares the data collected with a traditional EEG system (Study 1) to that collected with the RoBIK headset (Study 2). For all factors considered, data are collapsed across the other factors.