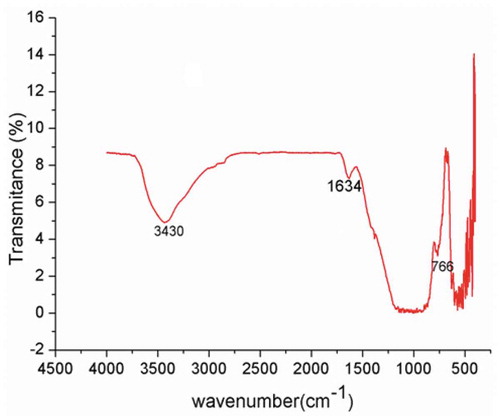
Figures & data
Table 1a. Elemental analysis of the VB before adsorption and after adsorption.
Table 1b. Elemental analysis of the VB before adsorption using EDS spectroscopy.
Figure 2. SEM images of VB before (a) and after adsorption (c) of Cr (III); EDS spectra before (b) and after adsorption (d) of Cr (III).
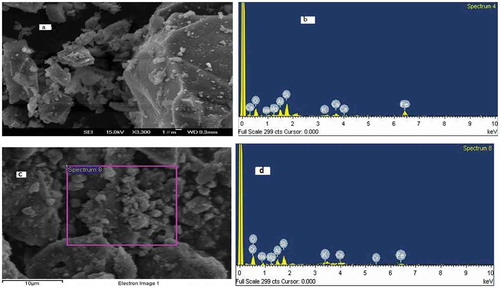
Figure 3. Effect of pH and ionic strength for Cr (III) adsorption onto the VB (dose 50 g L−1; Cr (III) conc. 100 mg L−1; temperature 25 ± 0.5°C) (a); Determination of pHpzc of Crushed VB in 0.01 mol L−1 KNO3 solution (b).
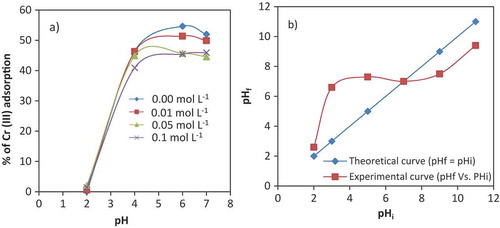
Figure 4. Effect of contact time on adsorption of different concentrations of Cr (III) ions (20, 60 and 100 mg L−1) on VB. (Adsorbent dose 50 g L−1, solution pH 6 and reaction temperature 25 ± 0.5°C).
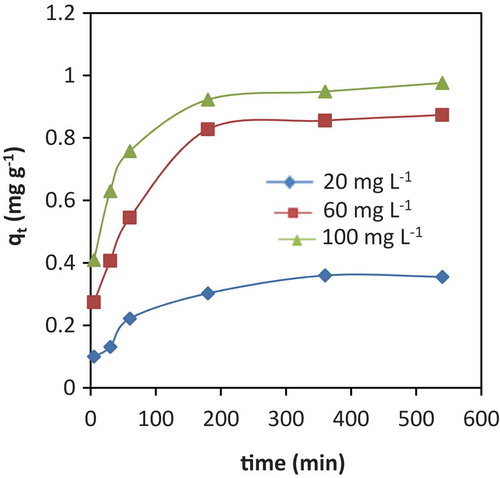
Table 2. Kinetic parameters for Cr (III) adsorption onto VB
Figure 5. Adsorption kinetics: pseudo-first order kinetic linear plot (a); pseudo-second order kinetic linear plot (b) and Elovich kinetic linear plots for adsorption of Cr (III) onto VB {initial concentration (20, 60 and 100 mg L−1), solution pH 6,adsorbent dosage 50 g L−1, temperature 25 ± 0.5°C)}.
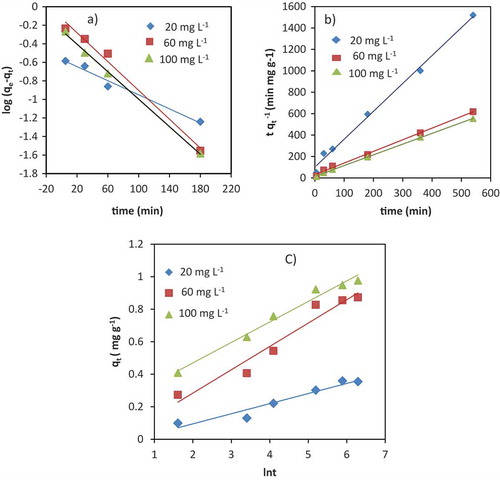
Table 3. Adsorption mechanism models for the adsorption of Cr (III) onto the VB
Figure 6. Linear plots of Intraparticle Diffusion (a), Bangham (b), and Boyd models (c) for adsorption mechanism of Cr (III) onto the VB rock.
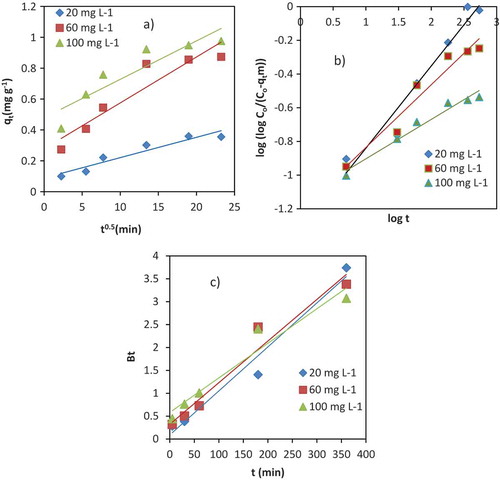
Table 4. Adsorption isotherm models
Figure 7. The fitting of Langmuir (a), Freundlich (b) and Temkin (C) isotherms of Cr (III) adsorption onto VB volcanic rock, pH 6, dose 50 g L−1.
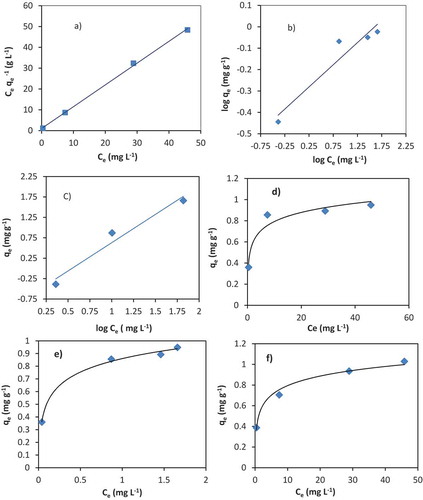
Table 5. Langmuir, Freundlich and Temkin isotherm parameters for adsorption of Cr (III) onto VB
Table 6. Comparison of adsorption capacity Cr (III) onto VB with other adsorbents