Figures & data
Table 1. All ingredients of sildenafil dry foam tablet formulation used in this study
Table 2. Finished product specification and physicochemical properties of sildenafil citrate dry foam tablets
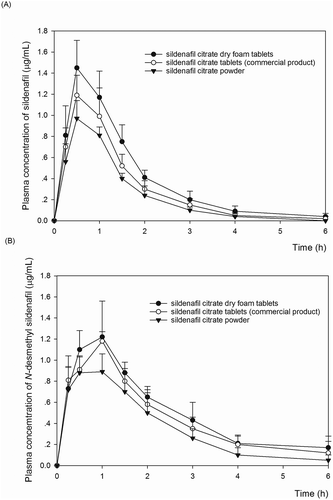
Table 3. Comparative mean (± SD) of pharmacokinetic parameters of sildenafil and N-desmethyl sildenafil in rats after oral administration
Figure 1. Mean plasma concentration—time profiles of sildenafil (A) and N-desmethyl sildenafil (B) after oral administration of sildenafil at a dose of 20 mg/kg in dry foam tablet (solid circles, n = 5), commercial film coated tablets (open circles, n = 5) and sildenafil powder (solid triangles, n = 5) in rats. Data are the mean ± standard deviation (SD).