Figures & data
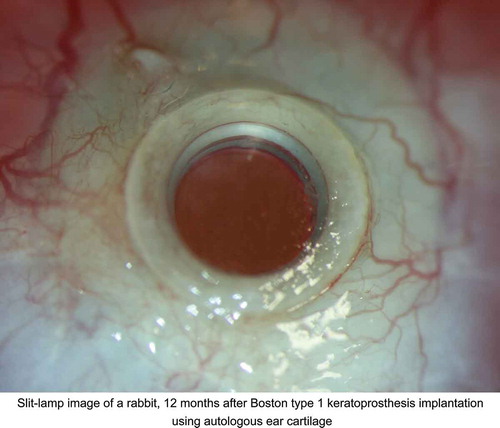
Figure 1. Implantation of the keratoprostheis (Kpro). A. Cartilage with perichondrium preserved; B. Thickness of the graft; C. Kpro with autologous ear cartilage as a carrier; D. Kpro with ear cartilage implanted in the left eye of the rabbit.

Figure 2. Schematical drawing of the surgical procedure. A. An 8.5 mm trephine is used to create a corneal pocket. B. The anterior chamber was irrigated with a heparin solution. C. The cornea-Kpro complex was placed in the recipient bed. D. The chondro-Kpro complex was placed in the recipient bed.
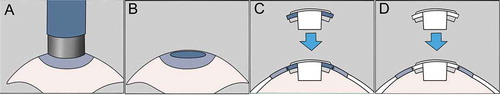
Table 1. Preoperative and postoperative variables
Figure 3. Implanted chondro-Kpro in rabbit 1. A. AS-OCT horizontal scan revealed a gap (arrow) on both sides between the posterior surface of the anterior plate and cartilage. B. The membrane over the anterior plate becomes translucent 8 months post-operation. C. AS-OCT horizontal scan showed that the gap between the anterior plate and cartilage was replaced by regenerative tissue; the gap (arrow) was only present on the left side, and the height decreased. D. After dissection of the granular tissue over the anterior plate 22 months post-implantation, no obvious retroprosthetic membrane was observed.
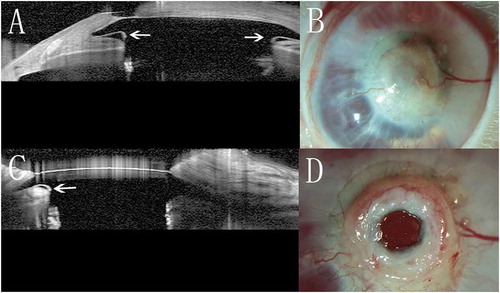
Figure 4. Light micrograph of the membrane over the anterior plate in rabbit 1. A. Fibrovascular tissue with eosinophils (white arrow), lymphocytes, fibroblasts and microvasculature (black arrow) in the anterior lay of the membrane (HE). B. Fibrovascular tissue in the medium lay of the membrane (HE). C. The inner layer of the membrane reveals the existence of lacunae (arrow) (HE). D. The inner layer of the membrane shows the existence of cartilage tissue (Alcian blue).
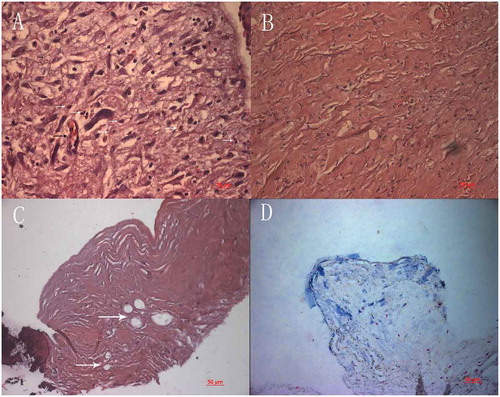
Figure 5. Light micrograph of the membrane over the anterior plate. Squamous epithelia adhere loosely to the fibrovascular tissue, Lacunae are demonstrated adjacent to the epithelial layer and over the optic (arrow, HE) .
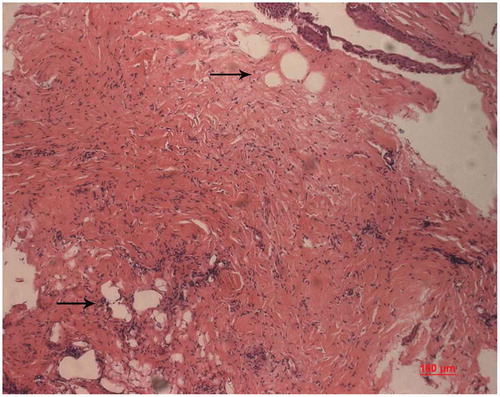
Figure 6. Internal growth of chondrocytes. The chondrocytes appear in groups and undergo cell division within their individual lacuna (arrow, HE) .
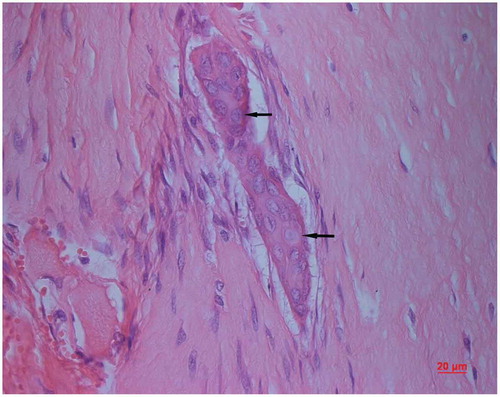
Figure 7. Implanted chondro-Kpro in rabbit 4. A. Light micrograph of the membrane over the anterior plate, eosinophils were demonstrated diffusely in the tissue (HE); B. Light micrograph of the membrane over the anterior plate, both collagen and keratin were demonstrated (Masson’s trichrome stain).
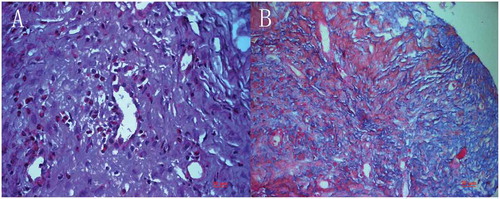
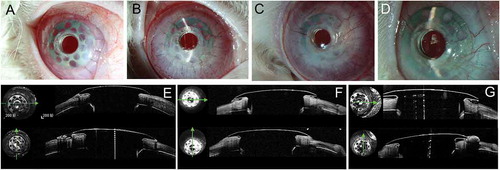
Figure 8. Implanted chondro-Kpro in rabbit 5. A. Left eye 3 days after Kpro implantation. B. Fibrovascular tissue was observed over the carrier cartilage 24 days postoperatively. C. Left eye 3 months after Kpro implantation. D. Left eye 8 months after Kpro implantation. No obvious RPM was observed. E. AS-OCT showed a gap between the carrier cartilage and posterior surface of the anterior plate 1 month postoperatively, and the gap was connected to the ocular surface. F. AS-OCT scan of the junction between Kpro and the cartilage 8 months postopertively; the gap disappeared, and the edge of the anterior plate was covered by epithelial tissue.
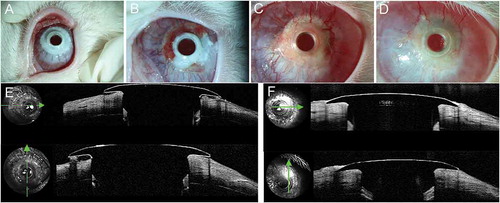
Figure 9. Implanted cornea-Kpro in rabbit 6. A. Left eye 1 month postoperatively, neovascularization was observed in the carrier corneal tissue from 2 o’clock to 3 o’clock . B. Left eye 2 months postoperatively. More vessel in the carrier cornea and air bubble beneath the flange. C. Left eye 3 months postoperatively. Neovascularization tissue could be seen beneath the flange and air bubbles disappeared. D. Left eye 8 months postoperatively. Air bubbles and debris beneath the flange. E. AS-OCT showed a small vertical gap between the anterior plate and carrier cornea 1 month postoperatively. F. AS-OCT showed tissue regeneration between the cornea and anterior plate of Kpro 3 months postoperatively. G; AS-OCT showed a gap between the cornea and posterior surface of the anterior plate 8 months postoperatively that connected to the ocular surface area on horizontal scan. On vertical scan, the corneal thinning was identified.