Figures & data
Figure 1. Social defeat stress-induced BAT thermogenesis and hyperthermia in rats. (A and B) Changes in BAT and abdominal (core) temperatures induced by social defeat stress (gray period with a horizontal bar). Changes from the temperature at time 0 are shown (n = 6). Temperature changes for 5 min after the beginning of the stress exposure are expanded in (B). (C) A site of muscimol nanoinjection in the rMR was labeled with fluorescent microspheres (arrow). py, pyramidal tract; RMg, raphe magnus nucleus; rRPa, rostral raphe pallidus nucleus. Scale bar, 500 µm. (D and E) Effect of muscimol or saline nanoinjection into the rMR (arrows) on social defeat stress-induced changes in BAT temperature (TBAT, D) and abdominal temperature (Tcore, E) (n = 5). Temperature changes from the baseline are shown (analyzed by 2-way ANOVA followed by Bonferroni's post-hoc test). **P < 0.01. All values are means ± SEM. Modified from Kataoka et al.Citation14 © Elsevier. Permission to reuse must be obtained from the rightsholder.
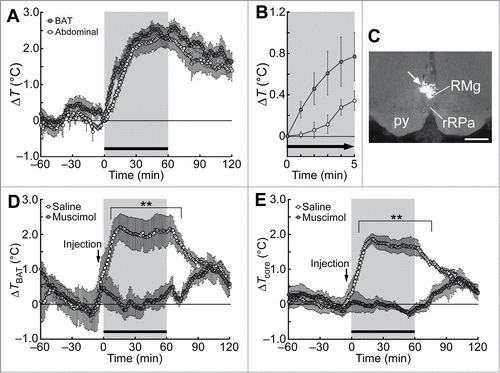
Figure 2. Social defeat stress activates rMR-projecting neurons and PVH-projecting neurons in the DMH. (A and B) Sites of injections of Alexa594-conjugated CTb (red) into the rMR (rMR-CTb, A) and Alexa488-conjugated CTb (green) into the PVH (PVH-CTb, B). Scale bars, 300 µm. (C and D) Fos immunoreactivity in CTb-labeled cells (arrows) in the dorsal part of the DMH (dDMH, C) and ventral part of the DMH (vDMH, D) following social defeat stress. Scale bars, 30 µm. (E and F) Distribution of rMR-CTb-labeled cells (red) and PVH-CTb-labeled cells (green) in the caudal hypothalamus of control (E) and stressed rats (F). Open and filled circles indicate cells negative and positive for Fos immunoreactivity, respectively. Very few cells were double-labeled with rMR-CTb and PVH-CTb (black circles). 3V, third ventricle; Arc, arcuate nucleus; cDMH, compact part of the DMH; f, fornix; ic, internal capsule; LH, lateral hypothalamic area; mt, mammillothalamic tract; ot, optic tract; VMH, ventromedial hypothalamic nucleus. Scale bar, 500 µm. Modified from Kataoka et al.Citation14 © Elsevier. Permission to reuse must be obtained from the rightsholder.
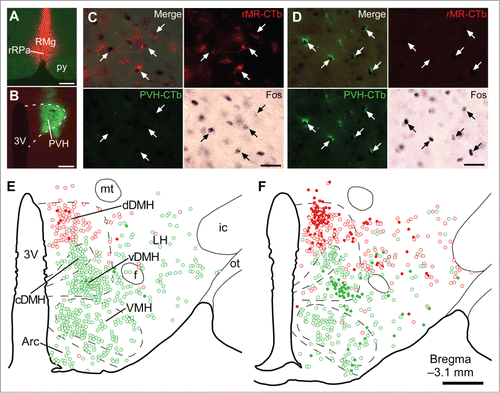
Figure 3. Optogenetic stimulation of DMH–rMR projection neurons elicits BAT thermogenesis and cardiovascular responses. (A) In vivo optogenetic experiment to selectively stimulate DMH-derived nerve endings in the rMR. (B and C) Cell bodies transduced with ChIEF-tdTomato (B, inset) in the DMH (mapped with circles) and their nerve endings containing ChIEF-tdTomato in the rMR (C). Scale bars, 500 µm (B); 30 µm (inset); 100 µm (C). (D) Effect of laser illumination of DMH-derived, ChIEF-tdTomato-containing nerve endings in the rMR on BAT thermogenic and cardiovascular activities. Horizontal bar, 30 sec. AP, arterial pressure; BAT SNA, BAT sympathetic nerve activity; HR, heart rate. Modified from Kataoka et al.Citation14 © Elsevier. Permission to reuse must be obtained from the rightsholder.
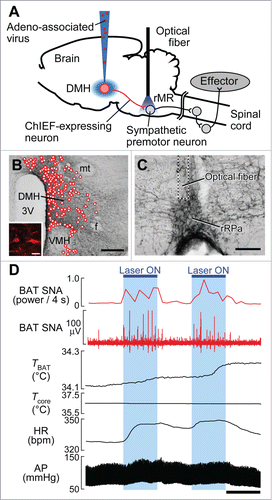
Figure 4. Schematic central circuits for sympathetic and neuroendocrine stress responses. Forebrain stress signals activate 2 groups of DMH neurons: dDMH neurons provide a direct glutamatergic input to sympathetic premotor neurons in the rMR to drive BAT thermogenesis and tachycardia contributing to PSH, and vDMH neurons provide a direct input to the PVH to drive a neuroendocrine outflow through the HPA axis to release stress hormones including glucocorticoids. Plus signs indicate excitatory neurotransmission. IML, intermediolateral nucleus. Modified from Kataoka et al.Citation14 © Elsevier. Permission to reuse must be obtained from the rightsholder.