Figures & data
Figure 1. Simplified model of organ temperature control by oxidative metabolism, the circulation and non-circulatory heat dissipation. The heat production in the organ is proportional to the arteriovenous difference in oxygen content. The amount of heat removed by the circulation is proportional to the blood flow and the temperature difference between the blood leaving the tissue (equal to the organ temperature, Torg) and the blood entering the tissue (core body temperature, Tc). The amount of heat dissipating to the environment is proportional to the difference between organ temperature and ambient temperature.
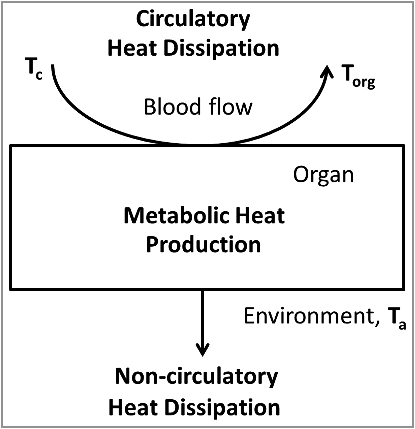
Figure 2. Comparison of brain and arterial blood temperature dynamics during body cooling induced by the consumption of cold food. A. A monkey fills cheek pouches with bananas chilled to 10°C and eats them (arrows). Note that the rapid cooling of arterial blood was followed by cooling of the frontal cortex and hypothalamus with a short delay. In contrast, the parietal subcortical white matter and subcutaneous tissue of the scalp demonstrated significant thermal inertia, as evident from the long delays in the response onset and nadir at each of these locations. B. A rabbit eats cold, chopped apples (arrows). The temperature dynamics in the aorta, basilar artery, and interpeduncular fossa were nearly identical (for clarity, the authors lowered the traces from the aorta and basilar artery), whereas those in the massa intermedia and ear skin were delayed. Replotted from the data reported in ref. [Citation22].
![Figure 2. Comparison of brain and arterial blood temperature dynamics during body cooling induced by the consumption of cold food. A. A monkey fills cheek pouches with bananas chilled to 10°C and eats them (arrows). Note that the rapid cooling of arterial blood was followed by cooling of the frontal cortex and hypothalamus with a short delay. In contrast, the parietal subcortical white matter and subcutaneous tissue of the scalp demonstrated significant thermal inertia, as evident from the long delays in the response onset and nadir at each of these locations. B. A rabbit eats cold, chopped apples (arrows). The temperature dynamics in the aorta, basilar artery, and interpeduncular fossa were nearly identical (for clarity, the authors lowered the traces from the aorta and basilar artery), whereas those in the massa intermedia and ear skin were delayed. Replotted from the data reported in ref. [Citation22].](/cms/asset/8fb908ea-ac62-421e-826c-54146fb75e4d/ktmp_a_1437311_f0002_b.gif)
Figure 3. Tail pinch induces an immediate increase of heat production in the brain, which is much shorter than systemic hyperthermia. A. Absolute temperatures reached their maximums in 10–15 min. B. The amplitude of temperature change (from prestimulation baseline) was higher in the brain than in the blood. C. Temperature differences between the blood and each brain structure are shown. The dynamics in the striatum is faster than in the cerebellum. D. The onsets of temperature responses to a tail pinch (first minute of responses shown in panel C). The data (mean ± SE) are replotted from ref. [Citation21].
![Figure 3. Tail pinch induces an immediate increase of heat production in the brain, which is much shorter than systemic hyperthermia. A. Absolute temperatures reached their maximums in 10–15 min. B. The amplitude of temperature change (from prestimulation baseline) was higher in the brain than in the blood. C. Temperature differences between the blood and each brain structure are shown. The dynamics in the striatum is faster than in the cerebellum. D. The onsets of temperature responses to a tail pinch (first minute of responses shown in panel C). The data (mean ± SE) are replotted from ref. [Citation21].](/cms/asset/1eba07ec-6e67-4250-bb3d-e5b36fc6b4a6/ktmp_a_1437311_f0003_oc.jpg)
Figure 4. Temperature differences between the arterial blood and various brain and subarachnoid sites in the monkey. The data are shown on two representative coronal sections, A (frontal coordinate of 14.3 mm) and B (frontal coordinate of 0.3 mm), adapted from Olszewski, 1952 [Citation38]. Note that, for the deep structures, the brain-blood temperature differences are in the range of 0.2-0.6°C. It is possible that non-circulatory dissipation to the environment is more significant in cortical structures and decreases the brain-blood temperature difference. The map of the brain-blood temperature differences is replotted from data reported in ref. [Citation23].
![Figure 4. Temperature differences between the arterial blood and various brain and subarachnoid sites in the monkey. The data are shown on two representative coronal sections, A (frontal coordinate of 14.3 mm) and B (frontal coordinate of 0.3 mm), adapted from Olszewski, 1952 [Citation38]. Note that, for the deep structures, the brain-blood temperature differences are in the range of 0.2-0.6°C. It is possible that non-circulatory dissipation to the environment is more significant in cortical structures and decreases the brain-blood temperature difference. The map of the brain-blood temperature differences is replotted from data reported in ref. [Citation23].](/cms/asset/21ae66df-7931-44ed-b627-b755631d18a5/ktmp_a_1437311_f0004_b.gif)
Figure 5. A genetically hairless rat (Crl:CD-Hrhr) exposed to cold. Two images of the animal are overlaid. The bottom layer is a regular (visible spectrum) photograph. The top layer is a semi-transparent, color-coded infrared thermogram. In the thermogram, temperatures from 31.0 to 37.0°C are coded with shades of yellow (from dark to light, respectively), temperatures below 31.0°C are coded with black, and temperatures above 37.0°C are coded with purple. As a result, the vasoconstricted hairless skin over the heat-exchange organs appears black, whereas the haired skin over the rest of the body is yellow. The external acoustic meatus and the skin over the interscapular brown adipose tissue have higher temperatures and show as purple. Reused from ref. [Citation41].
![Figure 5. A genetically hairless rat (Crl:CD-Hrhr) exposed to cold. Two images of the animal are overlaid. The bottom layer is a regular (visible spectrum) photograph. The top layer is a semi-transparent, color-coded infrared thermogram. In the thermogram, temperatures from 31.0 to 37.0°C are coded with shades of yellow (from dark to light, respectively), temperatures below 31.0°C are coded with black, and temperatures above 37.0°C are coded with purple. As a result, the vasoconstricted hairless skin over the heat-exchange organs appears black, whereas the haired skin over the rest of the body is yellow. The external acoustic meatus and the skin over the interscapular brown adipose tissue have higher temperatures and show as purple. Reused from ref. [Citation41].](/cms/asset/a0acdcf3-dcb1-4418-98f9-d4dadd0937b0/ktmp_a_1437311_f0005_c.jpg)
Figure 6. Temperature responses of a rat to ambient cooling. Exposure to 20°C induced cold thermogenesis (increased oxygen consumption), which resulted in increased colonic and muscle temperatures, despite the increased heat dissipation. Initially, the BAT temperature was slightly below the colonic temperature, but increased to 0.8°C above the colonic temperature during cold exposure. Replotted from data reported in ref. [Citation53].
![Figure 6. Temperature responses of a rat to ambient cooling. Exposure to 20°C induced cold thermogenesis (increased oxygen consumption), which resulted in increased colonic and muscle temperatures, despite the increased heat dissipation. Initially, the BAT temperature was slightly below the colonic temperature, but increased to 0.8°C above the colonic temperature during cold exposure. Replotted from data reported in ref. [Citation53].](/cms/asset/e11e800c-3661-438a-8f17-c7a6a5132434/ktmp_a_1437311_f0006_b.gif)
Figure 7. The BAT-blood temperature difference correlates with total body oxygen consumption. The graph combines experiments in unanesthetized rats exposed to 20°C (closed circles), with those exposed to one of 25 other ambient temperatures (open circles), with those exposed to 30°C and injected with an endotoxin that causes fever (crosses). Replotted from data reported in ref. [Citation49].
![Figure 7. The BAT-blood temperature difference correlates with total body oxygen consumption. The graph combines experiments in unanesthetized rats exposed to 20°C (closed circles), with those exposed to one of 25 other ambient temperatures (open circles), with those exposed to 30°C and injected with an endotoxin that causes fever (crosses). Replotted from data reported in ref. [Citation49].](/cms/asset/16a10e94-09f5-45d5-a27c-ced296e4ecf7/ktmp_a_1437311_f0007_b.gif)
Figure 8. Experimental manipulations can increase BAT temperature in an anesthetized, cooled rat preparation by more than 2°C. Replotted from data reported in ref. [Citation8].
![Figure 8. Experimental manipulations can increase BAT temperature in an anesthetized, cooled rat preparation by more than 2°C. Replotted from data reported in ref. [Citation8].](/cms/asset/fdce59e1-b3b2-4bfa-9e8b-086aa7ccd3cf/ktmp_a_1437311_f0008_b.gif)
