Figures & data
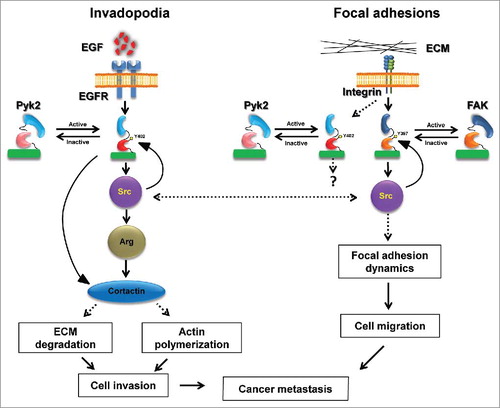
Figure 1. Pyk2 and FAK coordinate breast cancer cell invasiveness via distinct mechanisms. Following stimulation of epidermal growth factor receptor (EGFR), Pyk2 is recruited to the receptor and activated by auto-phosphorylation on tyrosine 402 (Y402). Activated Pyk2 recruits Src kinase, which leads to complete activation of Pyk2 as well as recruitment and activation of Arg. Both Arg and Pyk2 phosphorylate invadopodial cortactin, leading to MMP-dependent matrix degradation and actin polymerization in invadopodia, and consequent breast cancer cell invasion. At the same time, integrin activation in focal adhesions leads to recruitment and activation of FAK auto-phosphorylation on tyrosine 397 (Y397) and Src, which regulate focal adhesion dynamics by binding and phosphorylation of focal adhesion proteins. This signaling via FAK and Src leads to cancer cell motility-dependent invasiveness. A coordination of both Pyk2-mediated invasion and FAK-mediated migration is necessary for breast cancer cell invasiveness and consequent metastatic dissemination.