Figures & data
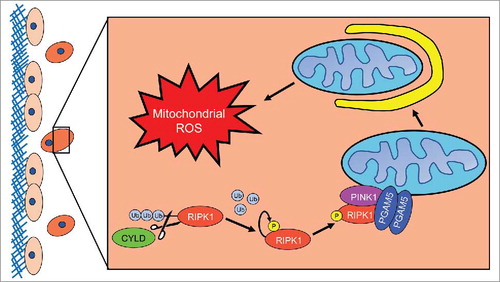
Figure 1. RIPK1 mediated induction of mitophagy during ECM detachment. When a cell detaches from extracellular matrix (ECM) (here depicted as the red cell detached from the blue ECM), Cylindromatosis (CYLD) will deubiquitinate receptor interacting protein kinase 1 (RIPK1) to promote its stability. Subsequently, RIPK1 will become fully active via autophosphorylation at S161. Next, RIPK1 will bind to and interact with phosphoglycerate mutase family member 5 (PGAM5) and PTEN-induced putative kinase 1 (PINK1). The formation of this complex will protect PINK1 from Presenilin associated rhomboid like (PARL) protease-mediated degradation, enabling PINK1 to promote mitophagy. As a consequence of mitophagy induction, ECM-detached cells experience diminished isocitrate dehydrogenase 2 (IDH2)-mediated NADPH production in the mitochondria and the subsequent elevation in mitochondrial ROS levels leads to non-apoptotic cell death.