Figures & data
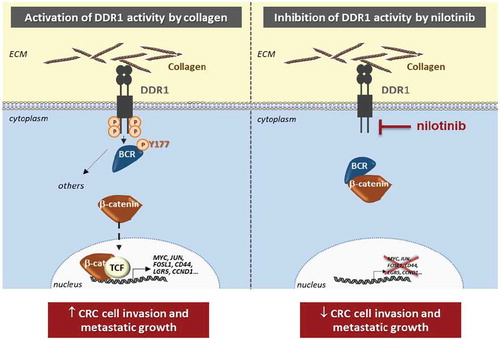
Figure 1. Inhibition of DDR1-BCR signaling by nilotinib. Collagens from the extracellular matrix (ECM) microenvironment of colorectal cancer (CRC) cells induce the kinase activity of DDR1 (Discoïdin Domain Receptor tyrosine kinase 1), which phosphorylates its substrate BCR (Breakpoint Cluster Region) on the tyrosine 177 (Y177), subsequently disrupting BCR/β-catenin interaction. This signaling cascade results in an increased β-catenin/TCF (T-Cell Factor) nuclear activity leading to the expression of critical target genes (including MYC, JUN, FOSL1, CD44, LGR5, CCND1) necessary for cell invasion and metastatic development (left panel). Inhibition of DDR1 kinase activity with nilotinib decreases CRC cell invasion and metastasis by reducing this β-catenin pathway (right panel).