Figures & data
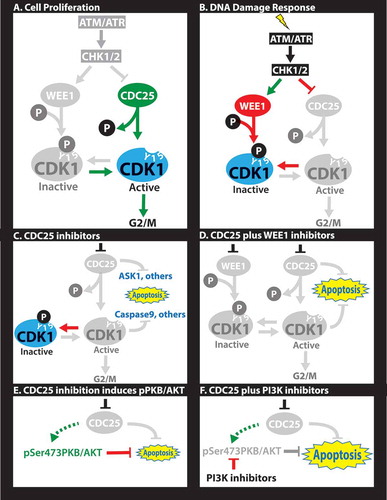
Figure 1. Molecular rationale for CDC25-targeting therapies. (A) In proliferating cells, CDC25 dephosphorylates Y15-CDK1 allowing for G2 to M progression. (B) In response to DNA damage, cells arrest at the G1/S (not shown) and/or G2/M transitions through activation of ATM/ATR, which induce CHK1/2, leading to phosphorylation of several targets. Phosphorylation of WEE1 stimulates this kinase to phosphorylate tyrosine (Y) 15 on CDK1 leading to cell cycle arrest. Phosphorylation of the CDC25 phosphatase inhibits its dephosphorylation of Y15-CDK1, further blocking G2/M progression. (C) CDC25 inhibitors unleash apoptotic pathways directly downstream of CDC25 (e.g. ASK1) or downstream of CDK1 (e.g. pCASPASE 9), leading to cell demise in diverse triple-negative breast cancers (TNBCs). (D) Combined treatment with CHK1 plus WEE1 inhibitors is synergistic through a mechanism yet to be established. (E) Sustained inhibition of CDC25 induces pSer473-PKB/AKT, likely as a feedback loop or epigenetic adaptation downstream of PI3K signaling that prolongs cell survival. (F) Combined treatment with CDC25 plus PI3K inhibitors is highly synergistic, leading to effective killing of TNBC.