Figures & data
Table 1. All the values are referred to transistors tested at room temperature for a fixed W/L geometry.
Figure 1. (a) A schematic representation of a neural interface divided in its main components; (b) some critical aspects to be considered for the designing of a performing neural interface.
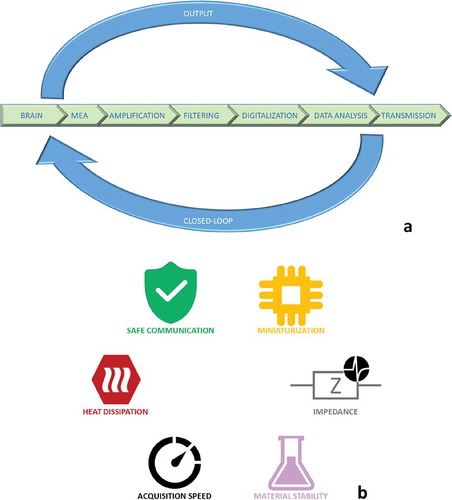
Figure 2. A sketch showing the parts of head where grids can be placed to collect signals from the cortex: subdural devices, epidural devices and penetrating electrodes. Non-invasive electrodes have not been taken into account in this scheme.
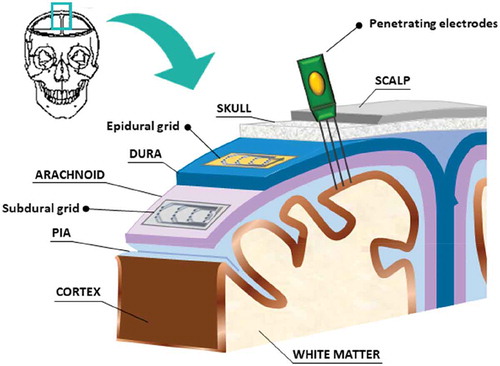
Figure 3. (a) A sketch showing the fundamental elements of a passive grid: a conductive film embedded into two layers of flexible polymeric materials; (b) the five properties that enhance grid functionalities to overcome challenges for a high-density recording/stimulating active grid.
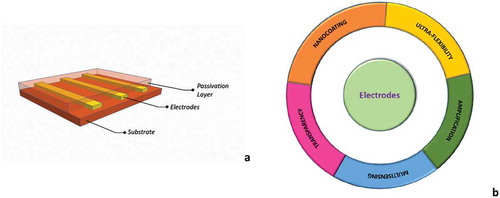
Figure 4. (a) Typical penetrating electrodes (Utah array); (b) Nanomesh electrodes copyright science advances [Citation89]; (c) Ultra-flexible polyimide-based MEAs covering the whole hemisphere of a rat brain, copyright scientific reports [Citation96]; (d) Syringe-injectable electronics copyright nature nanotechnology [Citation87]; (e) Pure PEDOT:PSS hydrogels copyright nature communications [Citation97]; (f) Electronic dura made in stretchable PDMS Copyright Science [Citation31] .
![Figure 4. (a) Typical penetrating electrodes (Utah array); (b) Nanomesh electrodes copyright science advances [Citation89]; (c) Ultra-flexible polyimide-based MEAs covering the whole hemisphere of a rat brain, copyright scientific reports [Citation96]; (d) Syringe-injectable electronics copyright nature nanotechnology [Citation87]; (e) Pure PEDOT:PSS hydrogels copyright nature communications [Citation97]; (f) Electronic dura made in stretchable PDMS Copyright Science [Citation31] .](/cms/asset/ab0560e8-fd34-4188-977e-dcea41121a95/tapx_a_1664319_f0004_oc.jpg)
Figure 5. (a) Flexible high-density active electrode array copyright nature neuroscience [Citation16]; (b) Organic electrochemical transistor–based electronics copyright nature communications [Citation151]; (c) Freely deformed stretchable array of CMOS inverters in PDMS copyright national academy of sciences 2008 [Citation28]; (d) Ultra-thin imperceptible e-skin copyright IEEE Spectrum [Citation120]; (e) Colorized SEM image of an epidermal electronic systems copyright advanced materials [Citation121] .
![Figure 5. (a) Flexible high-density active electrode array copyright nature neuroscience [Citation16]; (b) Organic electrochemical transistor–based electronics copyright nature communications [Citation151]; (c) Freely deformed stretchable array of CMOS inverters in PDMS copyright national academy of sciences 2008 [Citation28]; (d) Ultra-thin imperceptible e-skin copyright IEEE Spectrum [Citation120]; (e) Colorized SEM image of an epidermal electronic systems copyright advanced materials [Citation121] .](/cms/asset/654e775d-a8ea-4a56-9af3-9c2f51e1041d/tapx_a_1664319_f0005_oc.jpg)
Table 2. A list of the main features of the hybrid electronics systems used in neuroscience in terms of total bandwidth, sampling frequency, number of recording channels, power consumption, level of noise and ADC characteristics.
Figure 6. Possible technologies to be integrated in a future neural interface: local temperature and pH sensing, magnetic stimulation, drug delivery embedded functionalities, infrared-based communication module, reliable bidirectional communication and even brain-to-brain connectivity. Flexible electronics represents a promising ally to make it possible.

