Figures & data
Figure 1. The MSNs and carbon-based nanocarriers as versatile platforms for stimuli-responsive drug delivery in cancer theranostics. The time- and space- controlled drug release has been achieved by the employment of light, redox potential, pH gradient and magnetic field. These nanocarriers can be functionalized by (i) biocompatible polymers such as PEG for better blood circulation; (ii) gold nanoparticles or QDs as optical detection probes; (iii) FA, cell penetrating peptides or antibodies as cancer-specific ligands; and finally, (iv) DNA plasmids or small interference RNA (siRNA) for gene therapy.
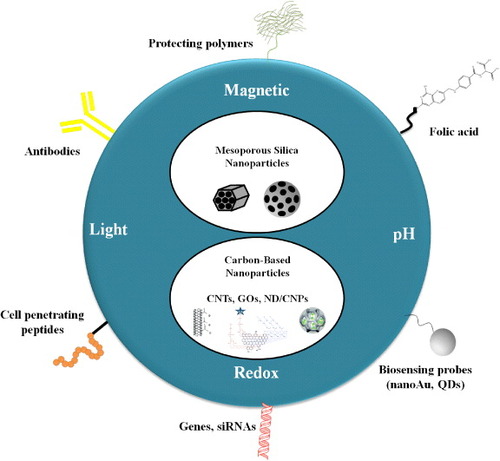
Figure 2. MCM-41 type MSNs were synthesized via self-assembly of silica and surfactant species. The cationic surfactants could self-assemble with negative-charged silica precursors to form ordered hexagonal mesoporous nanostructures. Surfactants can be removed via calcination.
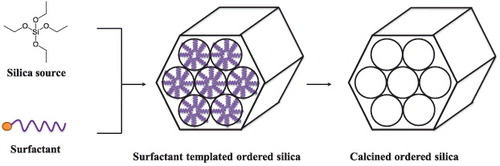
Figure 3. Schematic representation of the light-responsive MSNs. The metal core or shell of MSNs can effectively absorb NIR light and convert it to heat. With functionalization of thermal-responsive polymers on the surface of MSNs, drugs could be released upon remote NIR irradiation. Therapeutic drugs can also be immobilized on MSNs via photocleavable linkers. Upon irradiation at corresponding wavelengths, the linkers were cleaved and the cargos were unloaded from the porous structure of MSNs. Additionally, azobenzene derivatives, with their novel characteristics of the cis trans conformation switch by external light, could thereby be applied as ‘gatekeeper’ molecules to regulate the drug release from MSN-based nanocarriers.
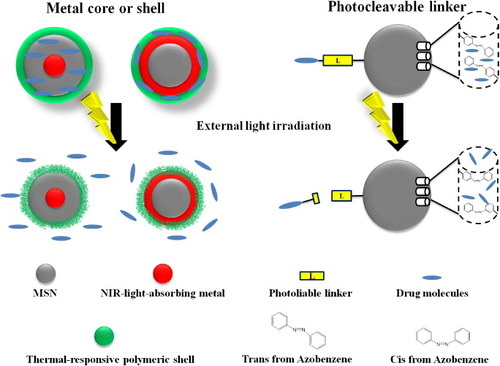
Figure 4. Schematic representation of the pH-responsive MSNs. Four major molecular designs are shown in the figure: (i) β-cyclodextrin and aromatic amine stalks could serve as effective ‘blockers’ to prevent leakage of drug molecules from nanocarriers. Under acidic pH, the amine stalks were protonated and the discharge of the β-cyclodextrin ring led to on-site drug release. (ii) Nanoparticles were modified on the surface of MSNs via acid-liable linkers to trap drug molecules in the silica mesoporous structures. Cleavage of these acid-sensitive linkers under acidic condition led to drug release. (Alternatively, direct modification of drug molecules with acid-liable linkers can also be achieved.) (iii) Many metal-containing compounds, especially transition metals, consist of coordination complexes. Drug molecules were conjugated with ligands or complexing agents, which enabled strong binding with the metal ion. The coordination bonding of the metal ion and its ligand is sensitive to the external pH and thereby the drug molecules were released. (iv) The dissoluble ZnO QDs could be conjugated on the MSNs to serve as the nanolids. The ZnO QDs were stable and insoluble under neutral pH and rapidly dissolved at a pH below 5.5. As a result, drug molecules can be released from MSNs due to the intracellular pH gradient.
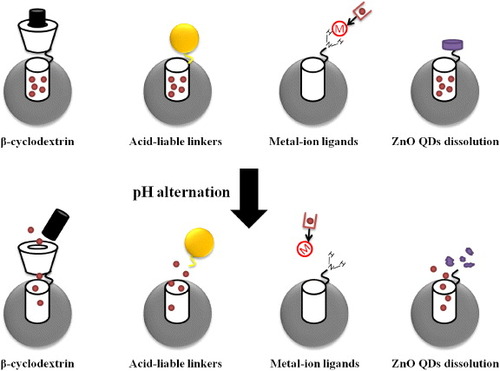
Figure 5. Schematic representation of the redox-responsive MSNs as a novel DDS in cancer cells. In the system, cargo molecules were often tethered in the cylindrical pores of MSNs via disulfide bond linkage or trapped by disulfide cross-linked polymers to prevent drugs leakage during the delivery routes. The cancer specific ligands enabled effective tumor targeting. Upon redox potential stimuli in the cancer cells, the cargo molecules then could be released.
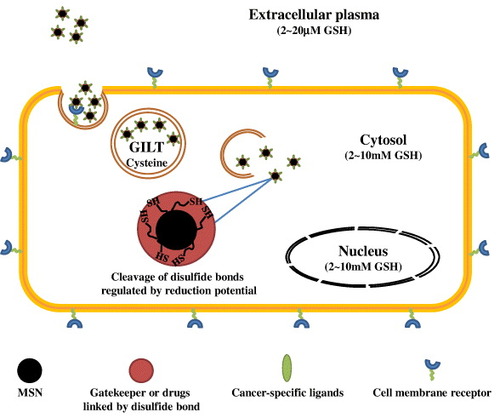
Figure 6. Schematic representation of magnetic field-responsive MSNs. A metal–chelator complex of gadolinium such as Gd-DTPA and manganese oxide nanoparticles have been applied as a preferred T1 contrast agent for MRI, while SPIOs and MNPs were commonly introduced as T2 contrast agents which provided dark, negative images as the intensity of the T2 signal increased.
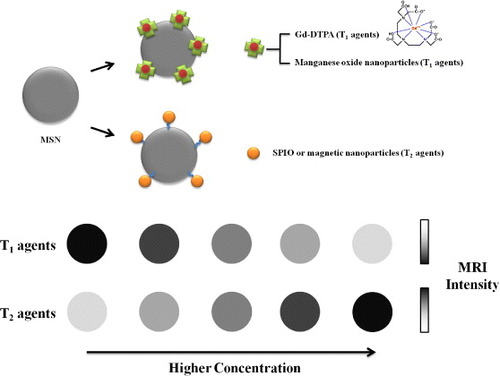
Table 1. Various stimuli-responsive MSNs-based DDS applied in cancer theranostics.
Table 2. CNT-based imaging/diagnostic systems in cancer theranostics.
Figure 7. Schematic of a tumor-targeted DDS that uses biotin-linker-taxoid functionalized SWNT nanoconjugates. A link to tumor-targeting modules as well as pro-drug modules has been demonstrated. The tumor-recognition modules were designed by the attachment of biotin and a spacer to the nanotube surface. The conjugation of pro-drug modules of an anticancer agent (taxoid with a cleavable S-S linker) were achieved, which could be activated to its cytotoxic form inside the cancer cells upon internalization and in situ drug release. Adapted with permission from Chen et al [Citation108]. Copyright© 2008, American Chemical Society.
![Figure 7. Schematic of a tumor-targeted DDS that uses biotin-linker-taxoid functionalized SWNT nanoconjugates. A link to tumor-targeting modules as well as pro-drug modules has been demonstrated. The tumor-recognition modules were designed by the attachment of biotin and a spacer to the nanotube surface. The conjugation of pro-drug modules of an anticancer agent (taxoid with a cleavable S-S linker) were achieved, which could be activated to its cytotoxic form inside the cancer cells upon internalization and in situ drug release. Adapted with permission from Chen et al [Citation108]. Copyright© 2008, American Chemical Society.](/cms/asset/a9493223-c067-4976-bdb4-8379980e18a2/tsta_a_11661384_f0007_oc.jpg)
Table 3. CNT-based nanocomposites in cancer theranostics: applications in chemo-, photothermal- and gene therapy.
Figure 8. Schematics of GNT-assisted photothermal (PT) and photoacoustic (PA) theranostics. (a) The synthesis of GNTs and the targeted delivery. (b) The principle of targeting endothelial LYVE-1 receptors with antibody–GNT complex (left panel) and PA (top right) and PT (bottom right) detection modules. GNTs conjugated with an antibody specific to the LYVE-1 receptor (anti-LYVE-1 antibody) were delivered to the lymphatic vessels and exposed to a laser pulse using an integrated intravital microscope. Laser-induced PA/PT effects at a relatively low laser energy were used for non-invasive diagnostics. For therapeutic purposes, such as ablating individual cells locally, the increased laser energy accompanied by microbubble formation could be applied. Adapted with permission from Kim et al [Citation82]. Copyright© 2009, Rights Managed by Nature Publishing Group.
![Figure 8. Schematics of GNT-assisted photothermal (PT) and photoacoustic (PA) theranostics. (a) The synthesis of GNTs and the targeted delivery. (b) The principle of targeting endothelial LYVE-1 receptors with antibody–GNT complex (left panel) and PA (top right) and PT (bottom right) detection modules. GNTs conjugated with an antibody specific to the LYVE-1 receptor (anti-LYVE-1 antibody) were delivered to the lymphatic vessels and exposed to a laser pulse using an integrated intravital microscope. Laser-induced PA/PT effects at a relatively low laser energy were used for non-invasive diagnostics. For therapeutic purposes, such as ablating individual cells locally, the increased laser energy accompanied by microbubble formation could be applied. Adapted with permission from Kim et al [Citation82]. Copyright© 2009, Rights Managed by Nature Publishing Group.](/cms/asset/0d0f646b-671b-4c97-8852-0ca51bb0f404/tsta_a_11661384_f0008_oc.jpg)
Figure 9. A CNTs-based gene delivery system fabricated by LbL assembling. The antisense oligodeoxynucleotides (ASODNs) as a therapeutic gene could easily be immobilized on the acid-treated CNTs via the coating of positively charged polymers at the interfaces. Additionally, fluorescent probes such as CdTe QDs could be labeled in ASODNs to track the uptake of materials. Reprinted (adapted) with permission from Jia et al [Citation86]. Copyright© 2007, American Chemical Society.
![Figure 9. A CNTs-based gene delivery system fabricated by LbL assembling. The antisense oligodeoxynucleotides (ASODNs) as a therapeutic gene could easily be immobilized on the acid-treated CNTs via the coating of positively charged polymers at the interfaces. Additionally, fluorescent probes such as CdTe QDs could be labeled in ASODNs to track the uptake of materials. Reprinted (adapted) with permission from Jia et al [Citation86]. Copyright© 2007, American Chemical Society.](/cms/asset/f2096dee-ac47-49ae-b0cc-a07f4c181d1d/tsta_a_11661384_f0009_oc.jpg)
Figure 10. The nanosized, reduced graphene oxide (nano-rGO) sheets with high NIR light absorbance and biocompatibility for potential photothermal therapy. The figure illustrates that the attachment of a targeting peptide bearing the RGD motif to nano-rGO afforded selective cellular uptake in cancer cells via αvβ3 integrin receptors recognition. In this nanosystem, stability was further improved by the noncovalent functionalization of amphiphilic PEGylated polymers on the nano-rGO sheets. Adapted with permission from Robinson et al [Citation128]. Copyright© 2011, American Chemical Society.
![Figure 10. The nanosized, reduced graphene oxide (nano-rGO) sheets with high NIR light absorbance and biocompatibility for potential photothermal therapy. The figure illustrates that the attachment of a targeting peptide bearing the RGD motif to nano-rGO afforded selective cellular uptake in cancer cells via αvβ3 integrin receptors recognition. In this nanosystem, stability was further improved by the noncovalent functionalization of amphiphilic PEGylated polymers on the nano-rGO sheets. Adapted with permission from Robinson et al [Citation128]. Copyright© 2011, American Chemical Society.](/cms/asset/44aa8c9b-1dc3-4acf-836e-cfffc95b0ad3/tsta_a_11661384_f0010_oc.jpg)
Figure 11. A structurally ordered MCN synthesized using a MCM-48 type MSN as the template. This MCN material could serve as a transmembrane delivery vehicle for the intracellular release of a cell membrane impermeable fluorescence dye, Fura-2, inside human cervical cancer cells (HeLa). Adapted with permission from Kim et al [Citation88]. Copyright© 2008, American Chemical Society.
![Figure 11. A structurally ordered MCN synthesized using a MCM-48 type MSN as the template. This MCN material could serve as a transmembrane delivery vehicle for the intracellular release of a cell membrane impermeable fluorescence dye, Fura-2, inside human cervical cancer cells (HeLa). Adapted with permission from Kim et al [Citation88]. Copyright© 2008, American Chemical Society.](/cms/asset/09fb8922-258f-4a89-90c1-bef7993dd741/tsta_a_11661384_f0011_oc.jpg)