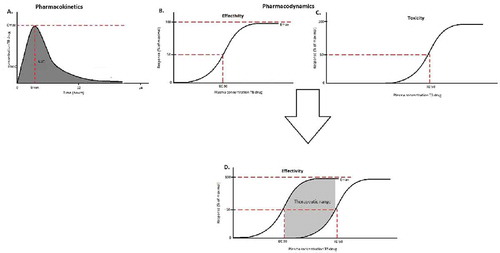
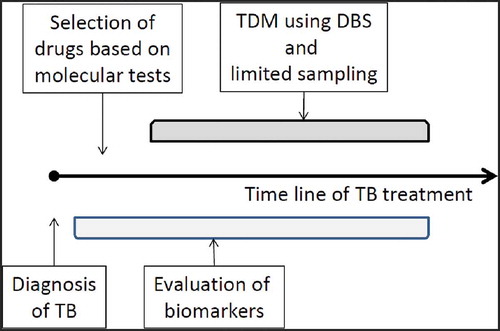
Figures & data
Table 2. Drug susceptibility test methods and critical concentrations for first- and second-line drug susceptibility tests together with molecular tests.