Figures & data
Figure 1. Stages of MCM development under the FDA Animal Efficacy Rule are shown. Important steps for developing MCM and FDA approvals are depicted in the above figure.
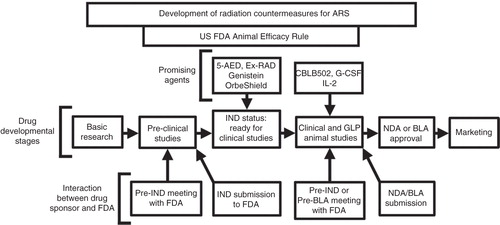
Figure 2. Terminology of therapeutic approaches in relation to radiation exposure is shown. Radioprotectors are administered before exposure and radiation mitigators are administered after radiation exposure during the prodromal/latent phase. Radiation therapeutics or treatments are given after symptoms manifest.
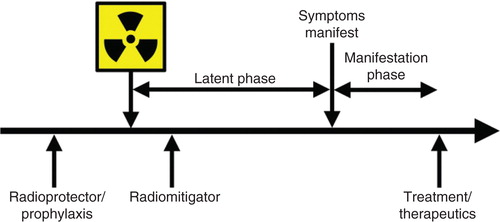
Figure 3. Evaluation of MCM for ARS in various animal models is shown. Those drugs that have been evaluated in multiple species are only mentioned under the highest animal model used for evaluation to date. Some drugs have been evaluated using additional models that are not mentioned here.
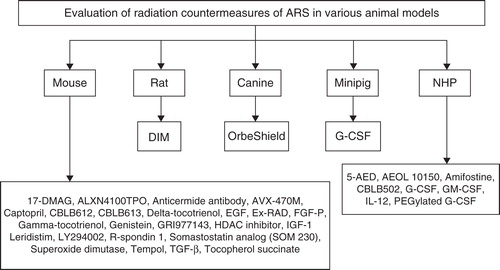
Table 1. Animal models of ARS for studying radiation injuries and development of medical countermeasures.
Figure 4. The effect of GT3 administration on sternal cellularity and megakaryocytes in irradiated mice is shown. GT3 was administered 24 h before irradiation. Mice were harvested 24 h post-irradiation for sterna. After fixation and processing, sternal bone marrow was stained (hematoxylin and eosin staining) to assess cellularity and megakaryocytes. Representative areas are shown above (100×, and an enlarged section of each photomicrograph at 400× magnification). GT3-treated sample has better cellularity compared with vehicle control.
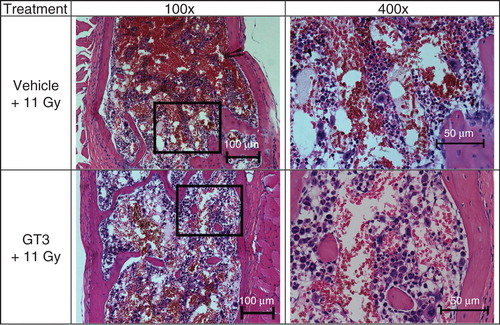
Figure 5. Effects of tocol treatment on bone marrow cellularity in irradiated nonhuman primates (NHPs) are shown. NHPs were treated with tocol or vehicle 24 h before 6.5 Gy whole-body 60Co γ-radiation. Sample of vehicle-treated NHP was collected on day 17 as a result of moribundity and tocol-treated NHP sample was collected on day 60 post-irradiation at the time of termination of the experiment. Sternum samples were collected, and after fixation were processed for hematoxylin and eosin stained sections to assess the cellularity. Representative areas are shown above (100×, and an enlarged section of each photomicrograph at 400× magnification). Tocol-treated NHP sample has better cellularity compared to vehicle control.
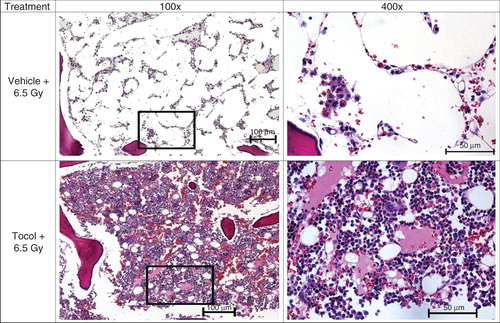
Figure 6. Effect of progenitor cells administered after irradiation on jejuna tissue area, crypt number, villus height and number, mitotic figures and length of basal lamina is shown. Photomicrographs of jejunal cross-sections are shown demonstrating the effect of progenitor cells administered 2 h after irradiation on jejuna tissue area, crypt number, villus height and number, mitotic figures per high power field and length of basal lamina containing five enterocytes 4 and 8 days after 13 Gy irradiation. CD2F1 male mice were irradiated with a dose of 13 Gy 60Co γ-radiation and transfused with 6 million progenitors or vehicle 2 h post-irradiation through para-orbital sinus injection. Tissue samples were harvested on days 4 and 8 post-irradiation, fixed and processed. Cross-sections of jejunum (4 µm) were stained (hematoxylin and eosin). Representative areas are shown (circumference at ×40, villus height at ×100, mitotic figure at ×1000 and basal lamina at ×600 magnifications) in photomicrographs: (A) circumference, (B) villus height, (C) mitotic figures and (D) basal lamina.
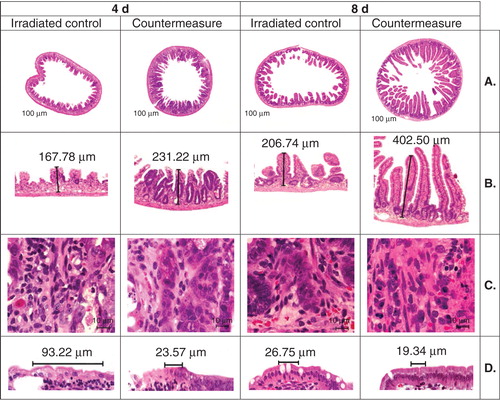
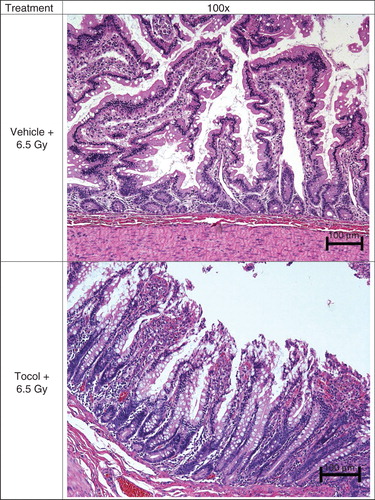
Figure 7. Effect of tocol treatment on jejunal tissue in irradiated nonhuman primates (NHPs) is shown. NHPs were treated with tocol or vehicle 24 h before 6.5 Gy whole-body 60Co γ-radiation. Jejunal samples of vehicle-treated NHP were collected on day 17 as a result of moribundity and tocol-treated NHP samples were collected on day 60 post-irradiation at the time of termination of the experiment. Tissue samples were fixed and processed for hematoxylin and eosin stained sections for assessment. Tocol-treated NHP jejunum has better structural organization cellularity compared with vehicle control.