Figures & data
A library of extracts (or fractions) are tested simultaneously against a DSBR-deficient cell line (e.g., a recB mutant of Escherichia coli) and a DSB-inducible cell line (e.g., the E. coli SbcCD/palindrome system [Citation9]). A WT E. coli cell line, which is unable to generate the inducible DSB, serves as a control for both screening assays. Extracts (or fractions) that exhibit ZOI against the DSBR-deficient cell line, but not the WT, are designated as inducers of DSBs. Similarly, extracts (or fractions) that exhibit ZOI against the DSB-inducible cell line, but not the WT, are designated as inhibitors of DSBR. The synergistic effect of these candidate extracts (or fractions) is validated by testing in unison against the WT cell line alone. The combination of inducers of DSBs and inhibitors of DSBR that generates ZOI against the WT cell line is expected to exhibit novel antimicrobial activity against pathogenic strains of E. coli. This cell-based screening model is also applicable to drug development against other pathogenic bacterial strains.
DSB: Double-strand break; DSBR: DSB repair; WT: Wild-type; ZOI: Zone of inhibition.
![Figure 1. Cell-based screening strategy for development of drugs targeting DNA double-strand break formation and repair in bacteria.A library of extracts (or fractions) are tested simultaneously against a DSBR-deficient cell line (e.g., a recB mutant of Escherichia coli) and a DSB-inducible cell line (e.g., the E. coli SbcCD/palindrome system [Citation9]). A WT E. coli cell line, which is unable to generate the inducible DSB, serves as a control for both screening assays. Extracts (or fractions) that exhibit ZOI against the DSBR-deficient cell line, but not the WT, are designated as inducers of DSBs. Similarly, extracts (or fractions) that exhibit ZOI against the DSB-inducible cell line, but not the WT, are designated as inhibitors of DSBR. The synergistic effect of these candidate extracts (or fractions) is validated by testing in unison against the WT cell line alone. The combination of inducers of DSBs and inhibitors of DSBR that generates ZOI against the WT cell line is expected to exhibit novel antimicrobial activity against pathogenic strains of E. coli. This cell-based screening model is also applicable to drug development against other pathogenic bacterial strains.DSB: Double-strand break; DSBR: DSB repair; WT: Wild-type; ZOI: Zone of inhibition.](/cms/asset/2b1e5356-fc74-4eed-9b72-b34b76fa8ea8/ifso_a_12364212_f0001.jpg)
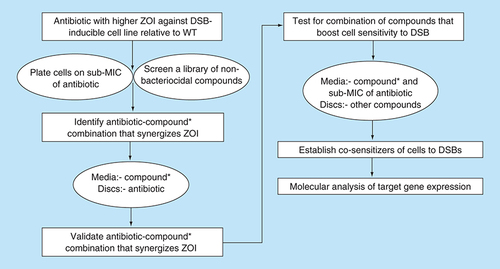
Antibiotics that exhibit higher zones of inhibition against the DSB-inducible cell line relative to wild-type are predicted to mediate inhibition of DSBR, thereby sensitizing cells to DSB formation. In order to boost this unique activity of the antibiotic, a library of nonbactericidal compounds is screened against the two cell lines following exposure to sub-MIC of the antibiotic. The nonbactericidal compounds that cause a further increase in the zones of inhibition are validated via a disc diffusion assay in which the agar plate is amalgamated with the compound and the antibiotics are infused onto discs. The procedure is repeated to demonstrate combinations of nonbactericidal compounds that exacerbate the sensitivity of cells to DSB formation in the presence of the antibiotic. Analysis of the expression profile of DSBR genes and associated stress response genes could provide valuable insight on the molecular mechanism underlying co-sensitization of cells to DSB formation. Importantly, the model could be incorporated into the cell-based screening assay, described in Figure 1, to identify extracts containing very low concentrations of inhibitors of DSBR.
*Nonbactericidal compound.
DSB: DNA double-strand break; DSBR: DSB repair; MIC: Minimum inhibitory concentration; ZOI: Zone of inhibition.
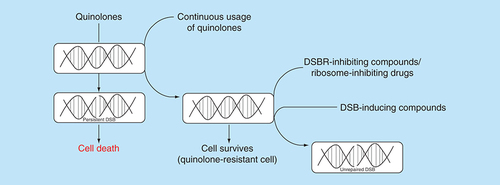
Quinolones generate persistent DSBs in bacteria and eventually cause cell death. The continuous usage of quinolones for treatment of bacterial infections has resulted in the emergence of strains which are resistant to the drug. A plausible approach for treatment of infections caused by quinolone-resistant strains is to administer compounds capable of inhibiting bacterial DSB repair in combination with novel DSB-inducing compounds. Alternatively, drugs that inhibit the bacterial ribosomes could be used to deplete global protein synthesis, including DSB repair proteins, thereby leading to accumulation of unrepaired DSBs in the quinolone-resistant strains.
DSB: DNA double-strand break; DSBR: DNA double-strand break repair.
(A) Profile of selected commercial antibiotics against Escherichia coli subjected (or not) to DNA double-strand breaks. Arrows indicate commercial antibiotics that modestly increased the sensitivity of E. coli to DNA double-strand breaks. These antibiotics are chloramphenicol (30 μg/μl), erythromycin (20 μg/μl), streptomycin (15 μg/μl) and gentamicin (10 μg/μl). (B) MIC determination for streptomycin on agar plates containing (or not) codeine. Escherichia coli::double-strand break represents an E. coli strain containing a system that induces a site-specific DNA double-strand break at the lacZ locus of the chromosome [Citation9]. Escherichia coli::NO double-strand break represents the control E. coli strain, which is unable to induce the site-specific DNA double-strand break at the lacZ locus.
DSB: DNA double-strand break; MIC: Minimum inhibitory concentration.
![Figure 4. Interaction of streptomycin with codeine enhances the susceptibility of Escherichia coli to DNA double-strand breaks.(A) Profile of selected commercial antibiotics against Escherichia coli subjected (or not) to DNA double-strand breaks. Arrows indicate commercial antibiotics that modestly increased the sensitivity of E. coli to DNA double-strand breaks. These antibiotics are chloramphenicol (30 μg/μl), erythromycin (20 μg/μl), streptomycin (15 μg/μl) and gentamicin (10 μg/μl). (B) MIC determination for streptomycin on agar plates containing (or not) codeine. Escherichia coli::double-strand break represents an E. coli strain containing a system that induces a site-specific DNA double-strand break at the lacZ locus of the chromosome [Citation9]. Escherichia coli::NO double-strand break represents the control E. coli strain, which is unable to induce the site-specific DNA double-strand break at the lacZ locus.DSB: DNA double-strand break; MIC: Minimum inhibitory concentration.](/cms/asset/b3c123bb-b7b1-46f6-b6d2-13e75fdce263/ifso_a_12364212_f0004.jpg)
