Figures & data
Table 1 Clinical features of the 24 female patients at baseline
Figure 1 Effect of niacinamide (n = 16), desonide (n = 16), and placebo (n = 16) on the luminosity change (L*Δ) in axillae treated during the study.
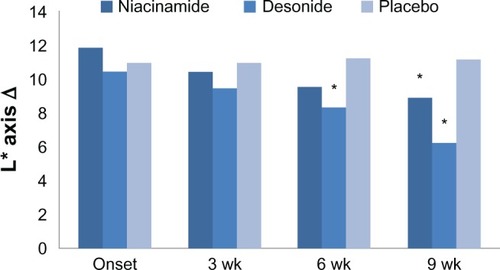
Figure 2 Physician assessment of improvement in axillary hyperpigmentation at the end of treatment (week 9).
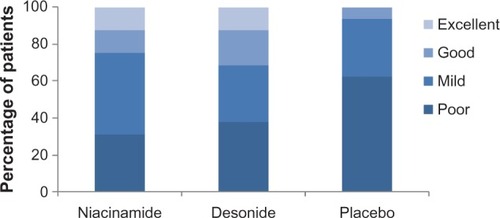
Figure 3 Photographs showing an excellent clinical response to treatment. (A) Axillae in a 22-year-old woman treated with niacinamide (left image). (B) The desonide-treated side in a 25-year-old woman at baseline and after 9 weeks of therapy (right image).
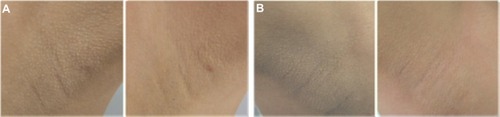
Figure 4 Reduction of epidermal melanin content (Fontana-Masson staining, 400×) after niacinamide and desonide, compared with placebo (first row).
Abbreviations: HE, hematoxylin and eosin staining; FM, Fontana-Masson staining.
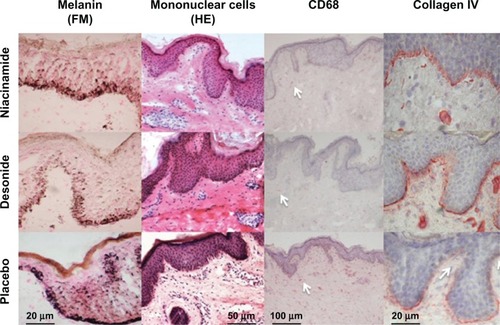
Table 2 Changes in colorimetric values (L*Δ, a*Δ), melanin content, mononuclear cells, NKI/Beteb, CD1a, CD68, collagen IV expression, and epidermal thickness in axillae treated with niacinamide, desonide, and placebo
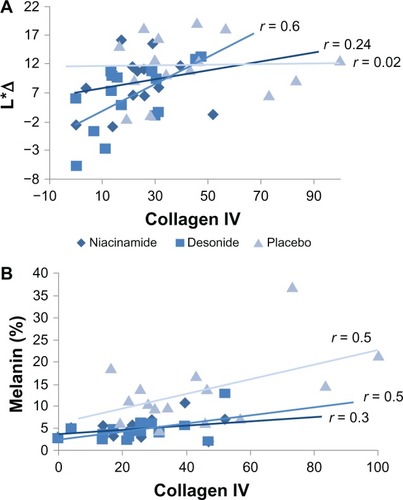
Figure 5 (A) Relationship between values in luminosity differences (L*Δ) of the affected axillae, and the percent of basal membrane discontinuity expressed as collagen IV staining for the interventions at the end of the study. A significant association was only evident for desonide (P = 0.01). (B) Relationship between melanin content by Fontana-Masson staining and collagen IV expression for each intervention at the conclusion of the study.