Figures & data
Figure 1 Schematic overview of unfolded protein response (UPR). When faced with stress conditions, the level of misfold or unfold proteins increases in the endoplasmic reticulum (ER) lumen and promotes recruitment of BiP. And then, three ER stress sensor IRE1, PERK, and ATF6 are activated. Active IRE1 triggers XBP1u mRNA, the unconventional splicing, to produce active transcription factor sXBP1. Active PERK promotes phosphorylation of elF2αleading to translation of ATF4 and CHOP. Translocation of ATF6 to the Golgi and proteolytic cleavage result in transcriptionally active form. Activation of these pathways trigger downstream transcriptional machinery resulting in expression of target genes associated with lipid metabolism, immune, inflammatory response, and differentiation, as well as structural/ functional expansion of ER and ERAD to overcome the stress conditions. Otherwise, persistent or excessive ERS might changes Ca2+ concentration inside mitochondria and mediate cell death pathway.
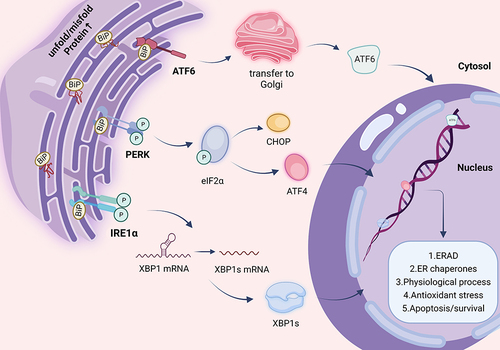
Figure 2 Pigmentary diseases related to ER homeostasis. Dysfunction of ER is associated with multiple diseases. Pigmentary disorders can manifest as abnormal pigmentation of the skin, eyes, and hair. These different kind of pigmentary diseases which are potentially relevant to ER dysfunction are listed.
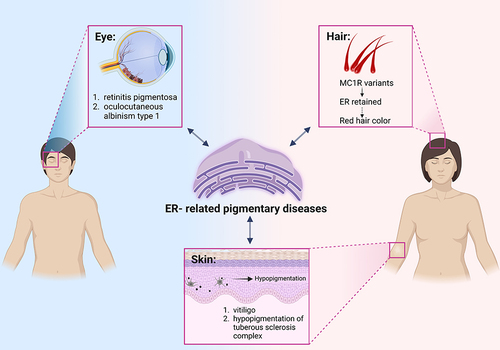
Figure 3 The cellular and intracellular interactions of ER in pathogenesis of vitiligo. The characteristic of vitiligo is melanocytes absence. Expanded ER can be seen in melanocytes and keratinocytes. The generation of ROS activates SIRT3-OPA1 and TRPM2, which cause Ca2+ released from ER to mitochondria. Both conditions cause mitochondrial fission and ultimately lead to apoptosis of melanocytes. The link between ER and melanosome might be associated with PMEL and melanin-related proteins in ER, such as tyrosinase ER retention. PMEL is synthesized in ER and ultimately transported melanosomes for fibril formation. But affected by Ca2+ homeostasis, improper maturation of PMEL fibrils and defected melanosome might also be involved in vitiligo. In addition, prolonged ERS could activate targeted genes and inflammatory reaction and increase the release of cytokines, which generates further ER stress and oxidative stress. CXCL16 could recruit CD8+ T cell, resulting in an anti-melanocyte autoimmune response.
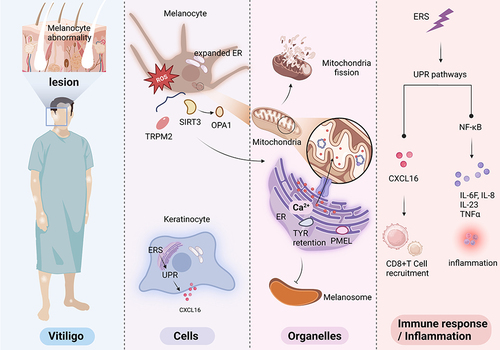
Table 1 Potential Therapeutic Drugs Targeted to ER in Diseases
