Figures & data
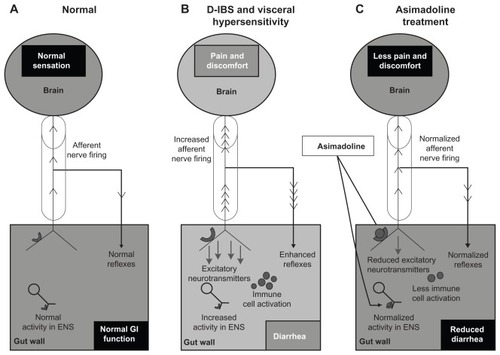
Figure 1 Effects of asimadoline on pain scores in D-IBS patients with at least moderate pain at baseline: asimadoline (asi) and placebo were administered twice daily for up to 12 weeks. Pain scores were collected daily and averaged numerically on a weekly basis. Week 0 represents the 2-week baseline period. As is apparent, with 0.5 mg and 1.0 mg dose levels, a substantial reduction in pain occurred, compared with placebo. Copyright © 2008. Reproduced with permission from Alimentary Pharmacology & Therapeutics. Mangel AW, Bornstein JD, Hamm LR, et al. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28(2):239–249.
Abbreviation: D-IBS, diarrhea-predominant irritable bowel syndrome.
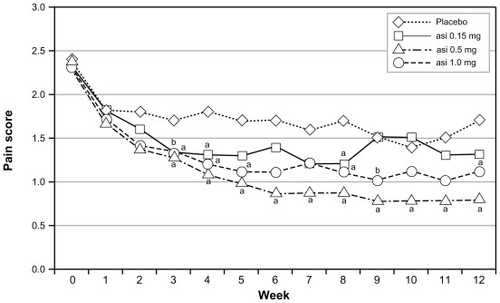
Figure 2 Effects of asimadoline (asi) on stool frequency in D-IBS patients with at least moderate pain at baseline: asimadoline (0.5 mg) and placebo were administered twice daily for up to 12 weeks. Stool frequency was collected daily and averaged numerically on a weekly basis. Week 0 represents the 2-week baseline period. As is apparent, with the 0.5 mg dose level a substantial reduction in stool frequency occurred, compared with placebo.
Abbreviation: D-IBS, diarrhea-predominant irritable bowel syndrome.
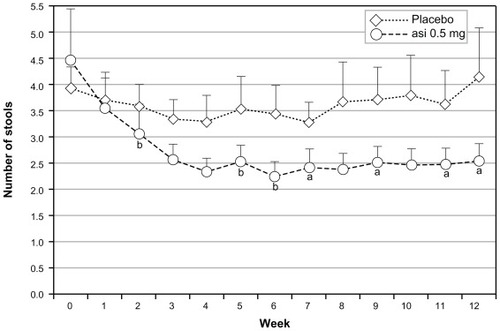
Table 1 Most common adverse events seen in nonirritable bowel syndrome asimadoline trials
Table 2 Most common adverse events in Phase IIb irritable bowel syndrome asimadoline trial among D-IBS patients with at least moderate pain at baseline
Figure 3 Proposed mechanism of action of asimadoline in the treatment of D-IBS. (A) The “brain–gut” axis in the normal state (health). Sensory information is conveyed to the brain via the spinal cord (cylinder) by extrinsic VAN. Local reflexes and descending regulatory input from the CNS via autonomic efferent nerves (not shown) control overall gut function. Normal secretomotor function is coordinated by the ENS. (B) Visceral hypersensitivity in D-IBS drives increased activity in VAN resulting in increased sensory signaling to the brain and enhanced local reflexes, contributing to diarrhea. The accompanying transmitter release into the gut wall results in increased ENS activity (leading to diarrhea) and immune cell activation, which heightens sensitization. Kappa receptor expression on VAN terminals is up-regulated due to sensitization. (C) Asimadoline activates kappa-opioid receptors on VAN, leading to reduced sensory input to the brain, reduced local reflexes, and reduced transmitter release. Asimadoline also acts presynaptically to reduce neurotransmitter release in the ENS. Pain, diarrhea, and visceral hypersensitivity are reduced.