Figures & data
Figure 1 Hepatic steatosis.
Abbreviations: ApoB, apolipoprotein B; FFAs, free fatty acids; FA, fatty acid; NAFLD, non-alcoholic fatty liver disease; VLDL, very-low-density lipoprotein.
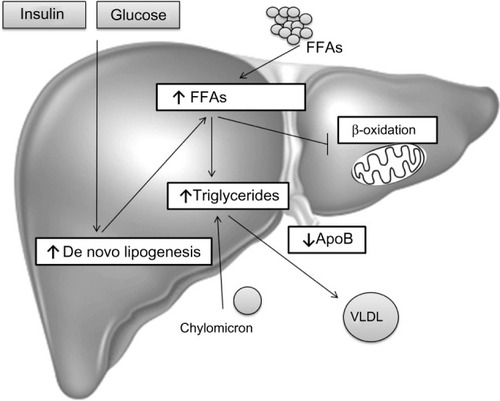
Figure 2 Transcriptional control of lipogenesis and glycolysis.
Abbreviations: ACC, acetyl-CoA carboxylase; ChREBP, carbohydrate-responsive element-binding protein; FA, fatty acid; FAS, fatty acid synthase; FFAs, free fatty acids; FxR, farnesoid X receptor; G6PC, glucose 6-phosphatase; GCKR, glucokinase regulatory protein; LXR, liver X receptor; MUFA, monosaturated fatty acids; PPARα, peroxisomal proliferator-activated receptor alpha; SCD1, steroyl CoA desaturase 1; SFA, saturated fatty acids; SREBP1c, sterol regulatory element-binding protein 1c; TG, triglyceride.
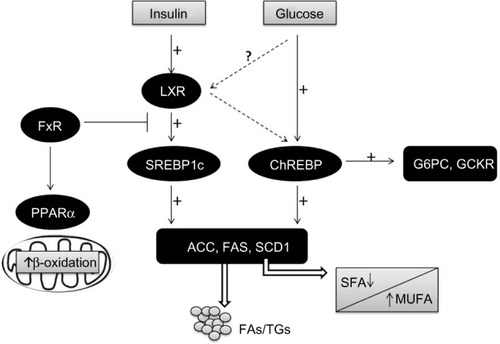
Figure 3 Fatty acid oxidation.
Abbreviations: CPT1, carnitine palmitoytransferase-1; FA, fatty acid; PPARα, peroxisomal proliferator-activated receptor alpha.
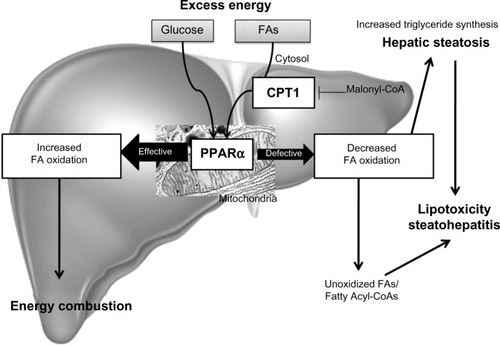
Figure 4 Cytokines and NAFLD.
Abbreviations: ADIPOR2, adiponectin receptor type 2; IL6, interleukin-6; NAFLD, non-alcoholic fatty liver disease; TNFα, tumor necrosis factor alpha; TNFR, tumor necrosis factor receptor; IL6R, interleukin-6 receptor.
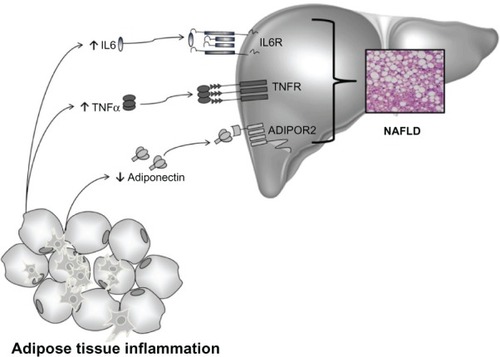
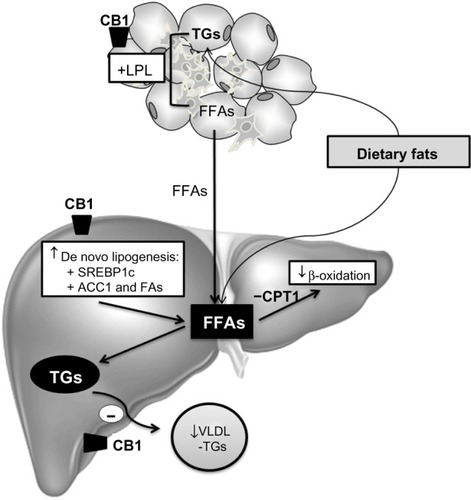
Figure 5 Mechanisms of CB1 involved in hepatic lipid accumulation.
Abbreviations: ACC1, acetyl-CoA carboxylase; CB, cannabinoid; CPT1, carnitine palmitoyltransferase-1; FA, fatty acid; FAS, fatty acid synthase; FFA, free fatty acid; LPL, lipoprotein lipase; SREBP1c, sterol regulatory element-binding protein 1c; TG, triglyceride; VLDL, very-low-density lipoprotein.