Figures & data
Figure 1 Calorie restriction (CR) impacts various cellular pathways and induces responses of the whole organism, leading to a more efficient metabolism, a higher protection against cellular damage, and the activation of remodeling mechanisms, whereas less efficient metabolism and synthetic pathways are blocked. CR inhibits processes involved in cell proliferation and glycolysis by blocking IGF-1 receptor-dependent pathways and TOR-dependent activities. CR exerts an anti-inflammatory effect by inhibiting nuclear factor-kB (NF-kB) activity. CR also decreases the production of ROS and increases mitochondrial biogenesis through different pathways (AMPK, sirtuins, and eNOS) leading to an improved mitochondrial physiology. The CR-induced activation of FoxOs implies the resumption of autophagy and mitophagy and the risen expression of antioxidants. CR also evokes activation of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) that increases the expression of mitochondrial and cell antioxidant enzymes. Any of these processes participates in the CR-related increase in improving healthspan and longevity.
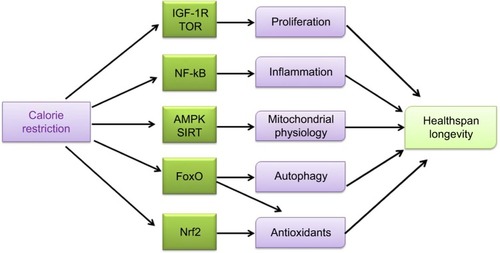
Figure 2 Molecular markers of aging involved in the pleiotropic effects of calorie restriction. Several markers characterizing aged cells are indicated by the affected molecules or functions. In the nucleus, aging implies the following: telomeres erosion, genomic instability, and epigenetic alterations (indicated by methylation [m] of histones H [Hm] and DNA [DNAm] or acetylation [ac] of histones [Hac]) with involvement of sirtuins and other modifying enzymes. In the mitochondria, age-related mitochondrial dysfunction leads to reduced ATP production and increased ROS presence. In the cytoplasm, age-dependent proteostasis imbalance causes an abnormal protein turnover with functional consequences. In the cytoplasm, aging also affects other pathways (eg, mTOR, IIS, AMPK, sirtuins, FoxOs) with dual effects namely on metabolism as well as on chromatin remodeling and regulation of gene expression, causing impaired nutrient/energy sensing that leads to different alterations, also due to reciprocal interrelationships.
![Figure 2 Molecular markers of aging involved in the pleiotropic effects of calorie restriction. Several markers characterizing aged cells are indicated by the affected molecules or functions. In the nucleus, aging implies the following: telomeres erosion, genomic instability, and epigenetic alterations (indicated by methylation [m] of histones H [Hm] and DNA [DNAm] or acetylation [ac] of histones [Hac]) with involvement of sirtuins and other modifying enzymes. In the mitochondria, age-related mitochondrial dysfunction leads to reduced ATP production and increased ROS presence. In the cytoplasm, age-dependent proteostasis imbalance causes an abnormal protein turnover with functional consequences. In the cytoplasm, aging also affects other pathways (eg, mTOR, IIS, AMPK, sirtuins, FoxOs) with dual effects namely on metabolism as well as on chromatin remodeling and regulation of gene expression, causing impaired nutrient/energy sensing that leads to different alterations, also due to reciprocal interrelationships.](/cms/asset/4ad989b3-24cf-4c53-ae3e-fe2674cc459f/dcia_a_126458_f0002_c.jpg)
