Figures & data
Figure 1 Mechanism of aging.
Notes: Superoxide dismutase, glutathione peroxidase, and catalase in the body eliminate oxygen free radicals. These enzyme’s activity is inhibited while aging and too many oxygen free radicals appear, leading to cell senescence. The autophagic lysosome formed by autophagic vesicles and lysosomes can encapsulate the molecules that need to be degraded in cells for degradation. The level of autophagy decreases with age. Telomerase can regulate the replication of telomeres and prevent them from shortening in chromosome replication, thus affecting aging. DNA methylation can reflect aging. The activation of the DNA damage response (DDR) in senescent cells promotes the attainment of proinflammatory secretory phenotype (SASP), which, in turn, triggers the activation of DDR and SASP in adjacent cells, thus creating a proinflammatory environment and accelerating senescence both locally and at the systemic level.
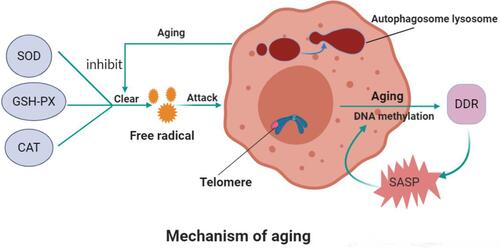
Figure 2 Mechanism of SOP.
Notes: With aging, the ability of BMSCs to differentiate into osteoblasts decreases and that of adipocytes increases. A decrease in peroxisome proliferator-activated receptor-γ coactivator 1 increases bone loss and fat accumulation during bone aging. Aging leads to the increase in reactive oxygen species (ROS), the accumulation of oxygen free radicals, the destruction of bone cells, and the inhibition of bone formation. With the growth of age, estrogen synthesis in vivo is reduced, CFTR chloride channel is lost, OPG is decreased, and overexpression of RANKL leads to osteoporosis. ClC-3 chloride channel in osteoblasts regulates the bone formation function of osteoblasts by regulating estrogen A receptor. In addition, the decrease in immune cells during aging leads to the decrease of OPG production and the increase in secretion of pro-inflammatory factors, which accelerates bone loss, affects bone metabolism and leads to osteoporosis. MiRNA networks can regulate bone formation through Wnt signaling pathway and bone resorption through RANKL/RANK pathway. In aging, autophagy level decreases and homeostasis of miRNA networks is broken. Finally, aging can down-regulate the expression and activity of 1α-hydroxylase in human bone marrow mesenchymal stem cells and inhibit the osteogenic differentiation of human bone marrow mesenchymal stem cells.
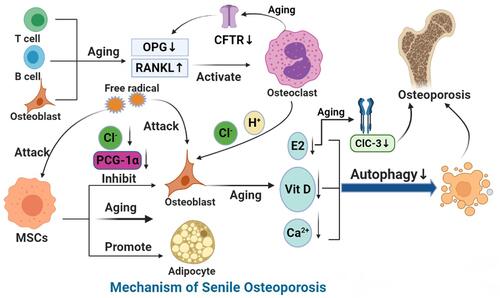
Table 1 Common Clinical Medications and Limitations in the Treatment of Osteoporosis
