Figures & data
Figure 1 NF1-OPGs in children. (A) A pre-chiasmatic OPG (red arrow) involving the left optic nerve. The optic nerve is enlarged and tortuous compared to the normal optic nerve on the right. (B) An OPG involving the optic chiasm. Red arrows point to the enlarged chiasm. (C) An OPG involving the bilateral optic radiations (red asterisks). The images are axial T2-weighted MRI scans.
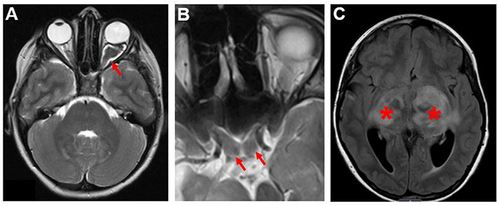
Figure 2 Proposed flow diagram for NF1-OPG screening and monitoring. Children with NF1 should undergo ophthalmological exam annually until 8 years of age, then every 2 years until age 18 if asymptomatic. Once symptoms develop, especially visual symptoms, MRI should be performed, and screening frequency should be increased to every 3 months for the first year. The intervals of the ophthalmologic examinations and MRIs can be gradually increased if stability of vision is achieved.
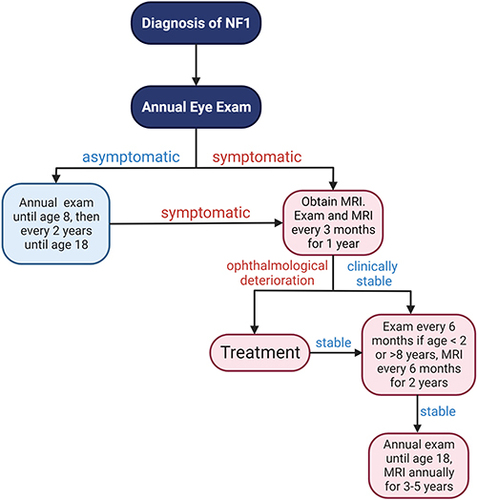
Figure 3 Interaction of tumor cells, neurons, and immune cells in murine Nf1-OPGs. Nf1-deficient tumor (glioma) cells produce Ccl2 to attract T cells, which release Ccl4 following exposure to Nf1-mutant neuronal midkine to induce Ccl5 secretion from Nf1-mutant microglia. Ccl5 functions as a potent mitogen for glioma cell growth. In addition, Nf1-mutant RGCs also promote tumor initiation and expansion through the elaboration of neuroligin-3 (Nlgn3) in response to light (visual experience). The establishment of a supportive microenvironment for Nf1-OPG formation and growth allows for risk factor convergence at the level of neurons (light, specific Nf1 mutation), T cells (asthma), and microglia (other systemic exposures).
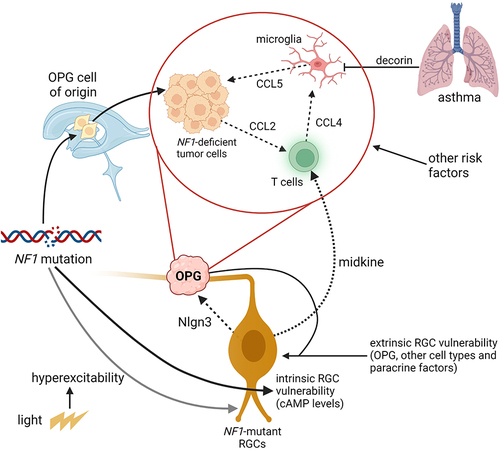
Table 1 Active Clinical Trials for NF1-OPG
