Figures & data
Table 1 Differential Diagnoses of SMARCB1 Deficient (Pediatric) CNS Tumors
Table 2 Overview of the Results of Clinical Phase III Trials and Consensus Regimens Exclusively in ATRT
Figure 1 Novel agents for ATRT therapy in clinical phase I and II trials - correlation to the tumor cell cycle. Arrows with even tips indicate “inhibition”, arrows with sharp tips indicate “activation”, fading arrows with sharp tips indicate “cell cycle exit into G0 phase”. Therapeutic approaches located in the center and surrounded by the grey circle affect more than one distinct phase of the cell cycle in the form of modified gene repression by epigenetically active drugs (decitabine (DNA-hypomethylation), tazemetostat (H3K27me3), entinostat and panobinostat (HDACI)), or DNA damage dependent cell cycle exit in G1 and G2 (radiotherapy induced double strand breaks). *Reaching and passing decision windows is driven by appropriate cyclin/CDK levels: G1/S transition is regulated by Cyclin D/CDK2/4/6, while Cyclin A/B/CDK1/2 affect G2/M transition.Citation86 A mitotic entry commitment point, a mitotic exit commitment point, or a S phase entry commitment point regulated by DNA damage, DNA replication stress, or spindle assessment are not shown in this figure. Created with Biorender.com.
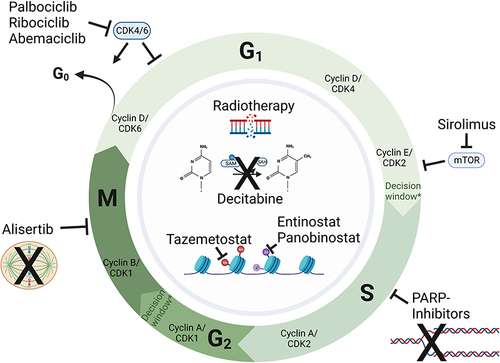
Figure 2 Novel targets affecting the interaction between ATRT tumor cells and the immune system. Structures investigated in clinical phase I and II trials targeting the interaction of T and ATRT tumor cells. Current clinical trials are provided for each agent in form of the NCT identifier. Trials including SMARCB1/SMARCA4-deficient entities only (focus on ATRT) are highlighted with *. Arrows with even tips indicate “inhibition”, arrows with sharp tips “uptake in”. Created with Biorender.com.
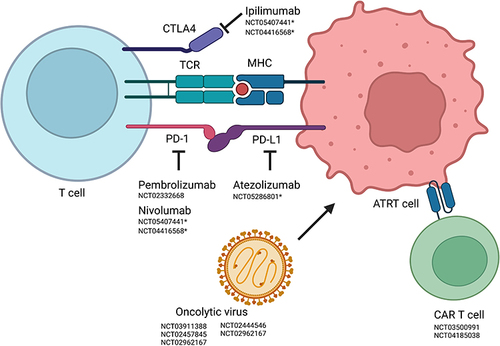
Table 3 Overview of Ongoing Clinical Trials Phases I and II Including Patients with ATRT
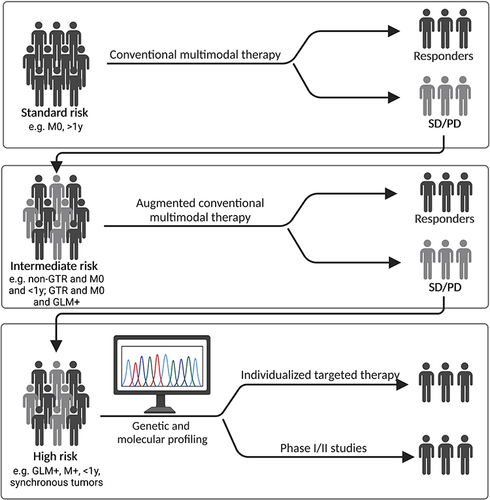
Figure 3 Risk factor-based model for treatment decision in ATRT. For standard risk patients, multimodal conventional therapy is the first line choice. Patients not achieving an objective response (SD, stable disease) and non-responders (PD, progressive disease) will be re-stratified into an intermediate risk group. Treatment of intermediate risk patients may add individual or combined novel targeting agents to conventional therapy. Delayed (SD) and non-responders (PD) will drop to a high-risk stratum. Treatment in the high-risk stratum will be individualized based on genetic and molecular profiling, and/or patients will be included in phase I/II clinical basket trials. Black characters represent responders, grey characters non-responders to current or previous treatment. Exemplary risk factors are provided for potential stratification and have to be defined by further research in form of international meta-analysis. Created with Biorender.com.