Figures & data
Figure 1 Cofilin participates in the “treadmilling” model of actin filaments. The combination of the abundant G-actin monomers provided by the G-actin pool with ATP can continuously bind to the barbed end (plus end) of F-actin to maintain the continuous growth of actin filaments. On the one hand, activated cofilin binds to ADP-actin on F-actin, cleaving and disaggregating actin filaments. Then, the dissociated ADP-G-actin quickly exchanges ATP and ADP to supplement G-actin monomer pool. On the other hand, cofilin can also bind to the dissociated ADP-G-actin, thereby preventing the exchange of nucleotides in the ADP-G-actin complex and inhibiting the polymerization of actin. LIMK1 can inhibit this process by phosphorylating cofilin. Conversely, SSH1 can dephosphorylate cofilin and activate it. Created with BioRender.com.
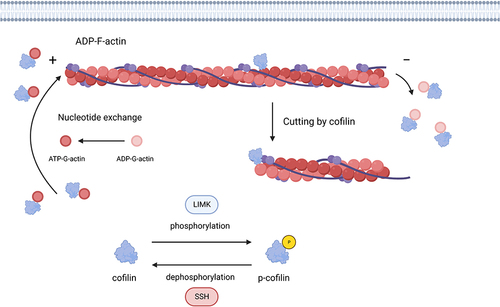
Table 1 Changes in Expression Levels of Cofilin in Tumor Cells and Their Correlated Phenotypes
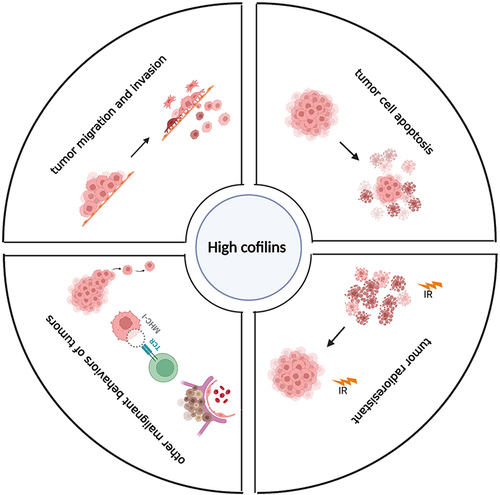
Figure 2 Cofilin regulates tumor migration and invasion represented by glioma. Dephosphorylating cofilin on Ser3 mediates its activation. Activated cofilin promotes tumor cell migration and invasion by regulating cell dynamics and EMT/EMT-like process. Cofilin can be inactivated through phosphorylation driven by LIMKs on Ser3 site, while SSH and CIN can dephosphorylate cofilin to restore its’ activity. LIMK is activated by the Rho-GTPases including Rac, CDC42 and Rho through their effector kinases, PAK1 and ROCK1/2 mediating the phosphorylation of LIMK on Thr508 resulting in its activation. Multiply proteins known to regulate the activity of cofilin via Rho-GTPases/ROCK&PAK/LIMK/cofilin signaling pathway resulting in regulating the actin-cytoskeleton dynamics, are SEP17, P2Y2R, NKCC1, CIRP8, Nogo-A, CD73, PRP4, AKT2, PKCζ, Cathepsin B, uPAR, P4, WDR1, Arp2/3. Some non-coding RNAs including miR451, miR29-A/b/c and ARST are also considered to participate in the regulation of cofilin through similar process. Non-SMAD signaling of TGF-β can induce rapid activation of RhoA, followed by phosphorylation and activation of LIMK2/cofilin1 pathway, resulting in regulating EMT/EMT-like process. Furthermore, TWIST1, KITENIN, VSIG4 and β-elemene are considered to be involved in the direct regulation of EMT/EMT-like process. Created with BioRender.com.
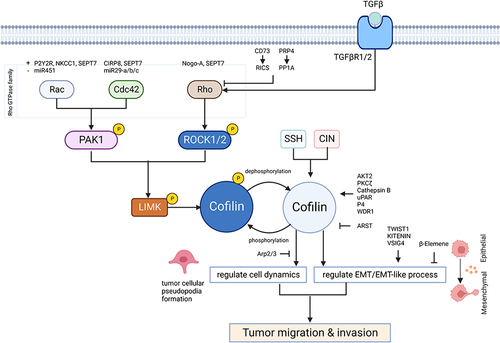
Table 2 Targets of Tumor Migration and Invasion Mediated by Cofilin
Figure 3 Cofilin regulates tumor cell apoptosis. Activated cofilin translocated to the mitochondria can directly bind to the potential cleavage sites in the outer mitochondrial membrane, which interacts with DRP1 to promote cytochrome C release and accelerate mitochondria breakage, resulting in tumor cell apoptosis. Created with BioRender.com.
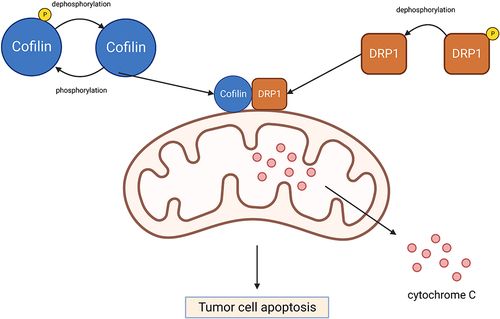
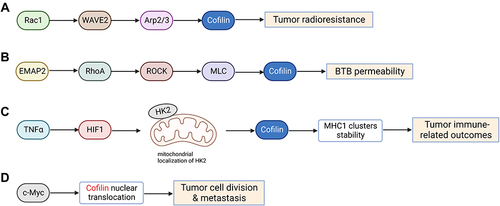
Figure 4 Cofilin regulates tumor radioresistance and other malignant behaviors. (A) Cofilin can enhance tumor radioresistance through activating Rac1-WAVE2-Arp2/3-Cofilin signaling pathway. (B) Low-dose EMAP-II promotes increased the activation of MLC and cofilin via RhoA/ROCK/MLC/Cofilin pathway, thereby triggering actin cytoskeleton dynamic remodeling and ultimately increasing the permeability of BTB. (C) HIF1α induced by TNFα impulses cofilin-mediated changes in actin filament dynamics to stabilize MHC-I clusters through affecting mitochondrial localization of HKII, eventually affecting tumor immune-related outcomes. (D) Activation of c-Myc promotes nuclear translocation of cofilin and F-actin cytoskeleton dynamic remodeling, which may in turn affect tumor cell division and metastasis. Created with BioRender.com.