Figures & data
Figure 1 Regorafenib inhibits oncogenesis, angiogenesis, and the tumor microenvironment in metastatic colorectal cancer. Regorafenib is a multikinase inhibitor that targets several receptor tyrosine kinases (eg, fibroblast growth factor receptor [FGFR], platelet-derived growth factor receptor-β [PDGFR-β], vascular endothelial growth factor receptor [VEGFR], and tyrosine kinase with immunoglobulin and epidermal growth factor homology domain [TIE2]) that regulate angiogenesis and the tumor microenvironment in colorectal tumor cells. Regorafenib also inhibits oncogenesis, in part, by selectively targeting the intracellular kinases BRAF and RAF1, an activator of downstream mitogen-activated protein kinases (MEK and ERK) that promote cell proliferation.
Abbreviation: ANG-2, angiopoietin-2.
![Figure 1 Regorafenib inhibits oncogenesis, angiogenesis, and the tumor microenvironment in metastatic colorectal cancer. Regorafenib is a multikinase inhibitor that targets several receptor tyrosine kinases (eg, fibroblast growth factor receptor [FGFR], platelet-derived growth factor receptor-β [PDGFR-β], vascular endothelial growth factor receptor [VEGFR], and tyrosine kinase with immunoglobulin and epidermal growth factor homology domain [TIE2]) that regulate angiogenesis and the tumor microenvironment in colorectal tumor cells. Regorafenib also inhibits oncogenesis, in part, by selectively targeting the intracellular kinases BRAF and RAF1, an activator of downstream mitogen-activated protein kinases (MEK and ERK) that promote cell proliferation.Abbreviation: ANG-2, angiopoietin-2.](/cms/asset/fa235ea5-eee2-4cb4-8da3-3a3b2cc1408e/dcmr_a_52217_f0001_b.jpg)
Figure 2 Kaplan–Meier curves for overall survival (A) and progression-free survival (B) in the study population.
Notes: The hazard ratio (HR) for overall survival at final analysis was 0.79 (95% CI: 0.66–0.94; P=0.0038). Reprinted from The Lancet, 381, Grothey et al, Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial, 303–312, Copyright © 2013, with permission from Elsevier.Citation11
Abbreviations: CI, confidence interval; IQR, interquartile range; PFS, progression-free survival.
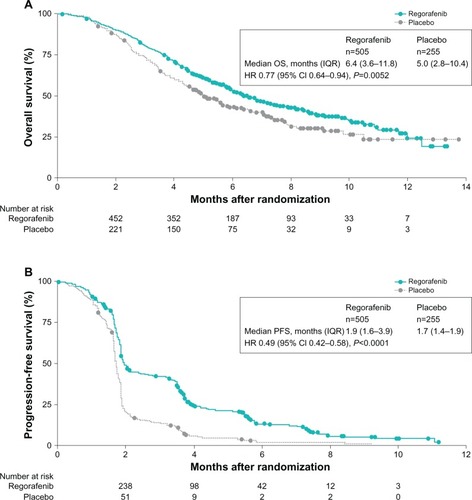
Table 1 Incidence of adverse events (≥10%) reported in patients treated with regorafenib in the CORRECT study and reported more commonly than in patients receiving placebo
Table 2 Incidence of liver function test abnormalities for patients in the safety population who had liver function tests
Figure 3 Time course of treatment-emergent adverse events in the CORRECT trial.
Note: Shown is the incidence of adverse events (AEs) over eight 4-week treatment cycles (one treatment cycle was 3 weeks of regorafenib 160 mg [or matching placebo] orally once daily and 1 week off treatment).
![Figure 3 Time course of treatment-emergent adverse events in the CORRECT trial.Note: Shown is the incidence of adverse events (AEs) over eight 4-week treatment cycles (one treatment cycle was 3 weeks of regorafenib 160 mg [or matching placebo] orally once daily and 1 week off treatment).](/cms/asset/3778c528-58ef-4063-8247-f6f3675ae686/dcmr_a_52217_f0003_c.jpg)
Table 3 Guidelines for modifying the regorafenib dose
Table 4 Management of regorafenib-related liver function abnormalities: grading of abnormalitiesTable Footnotea
Table 5 Management of regorafenib-related liver function abnormalities: dose-modification guidelines
