Figures & data
Figure 1 Study design for V2 and V3 during which one of the study medications (ipratropium bromide [500 µg]/salbutamol [2.5 mg] [Combivent®] or placebo) was administered.
![Figure 1 Study design for V2 and V3 during which one of the study medications (ipratropium bromide [500 µg]/salbutamol [2.5 mg] [Combivent®] or placebo) was administered.](/cms/asset/fae42347-385b-42f6-bdcc-cac68dd01f1f/dcop_a_113113_f0001_b.jpg)
Table 1 Baseline characteristics of the study population (n=40)
Table 2 Physiological and perceptual responses at the symptom-limited peak incremental cycle exercise (n=40)
Table 3 Effects of nebulized ipratropium bromide (500 µg)/salbutamol (2.5 mg), and 0.9% saline placebo on spirometry parameters at rest (n=40)
Figure 3 Effect of nebulized fixed-dose combination of ipratropium bromide (500 µg)/salbutamol (2.5 mg) (Combivent®) and 0.9% saline placebo on Borg 0–10 scale intensity ratings of dyspnea at the end of the third minute of exercise.
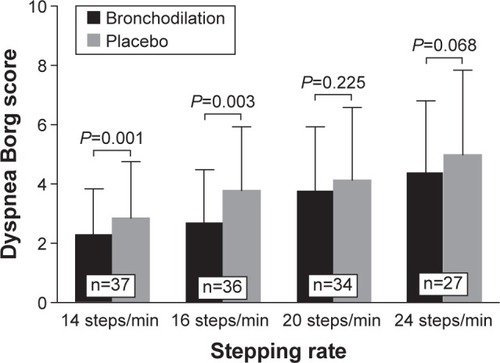
Table 4 Effect of single-dose inhalation of nebulized ipratropium bromide (500 µg)/salbutamol (2.5 mg) vs 0.9% saline placebo on physiological and perceptual responses at the end of the 3-MST, performed at 14, 16, 20 and 24 steps/min