Notes: RB in mice: (
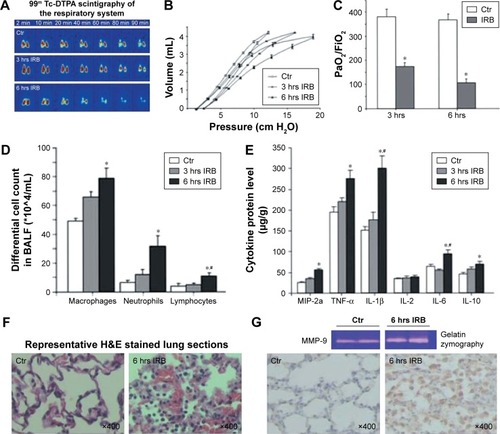
A) Tracheal banding induces lung injury. Lung histological evaluation by light microscopy revealed the existence of neutrophil infiltration in mice 24 hours after tracheal banding. RB increases interstitial and intraalveolar infiltration and focal congestion in the lung tissue. Representative histological section stained with hematoxylin and eosin of quietly breathing (control) and treated mice, after tracheal banding, respectively (upper panel). (
B) Tracheal banding induces inflammatory cells influx in the BALF. Total cells and macrophages in BALF of TB mice are increased compared to sham-operated mice (control) following 24 hours of resistive breathing. Respiratory system mechanics: (
C) Airway resistance by force oscillation technique is elevated following tracheal banding. (
D) The expression of sGC is decreased in the lungs of TB mice. Protein levels of sGC subunits are decreased in mice after 24 hours of resistive breathing. Sham-operated (control) or TB mice were sacrificed 24 hours after treatment. Representative Western blots for α-1, β-1, and β-actin are presented (upper panel). Blots were quantified by densitometry. Expression for each subunit normalized for β-actin was set at 100% for sham-operated mice. (
E) Therapeutic activation of sGC attenuates lung injury caused by tracheal banding. Lung histological evaluation by light microscopy revealed the attenuation of inflammation and neutrophil infiltration in mice treated with BAY58-2667. BAY58-2667 decreased interstitial and intraalveolar infiltration and focal congestion in the lung tissue, compared to the group of TB mice. (
F) Activation of sGC improves lung mechanics after tracheal banding. Elevated airway resistance, following tracheal banding, was attenuated when BAY58-2667 was administrated after TB. Values are expressed as mean ± SEM; n=10; *
P<0.05 for sham-operated (control) group and
#P<0.05 for TB + BAY58-2667 group. Figures
A–
F have been reprinted with permission of the American Thoracic Society. Copyright © 2016 American Thoracic Society. Glynos C, Toumpanakis D, Loverdos K, et al. Guanylyl cyclase activa tion reverses resistive breathing-induced lung injury and inflammation.
Am J Respir Cell Mol Biol. 2015;52:762–771.
Citation23 The American Journal of Respiratory Cell and Molecular Biology is an official journal of the American Thoracic Society. Smoking mice: (
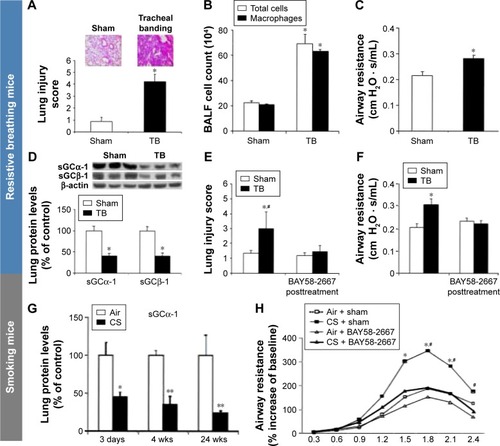
G) sGC expression is decreased in mice after cigarette smoke (CS) exposure. Protein levels of sGCα-1 upon acute (3 days), subacute (4 weeks), and chronic (24 weeks) CS exposure. Example of Western blot for sGCα-1 is presented (upper panel). Western blot was performed on eight mice per group. Blots were quantified by densitometry. Expression for the sGCα-1 subunit normalized for β-actin was set at 100% for air-exposed mice. Values are expressed as mean ± SEM; n=8/group; *
P<0.05, **
P<0.01 for air-exposed mice. (
H) BAY58-2667 administration attenuates airway hyperresponsiveness on acute CS exposure. BAY58-2667 administration in acute CS-exposed mice ameliorates bronchoconstriction. Effect of BAY58-2667 on percent increase in airway resistance (R) in a dose–response manner to serotonin (5-HT) challenge is presented. Values are expressed as mean ± SEM; n=8/group; *
P<0.05 for air-exposed group and
#P<0.05 for CS + BAY58-2667 group. COPD patients and smokers: (
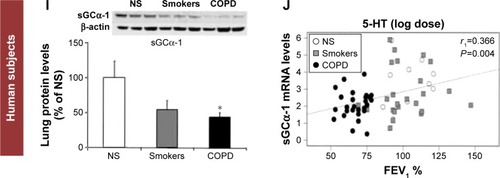
I) sGC protein expression is decreased in patients with COPD. Western blot was performed on a total of 33 patients (nine never smokers, nine smokers, and 15 patients with COPD). Examples of Western blots for sGCα-1 and β-actin are presented (upper panel). Expression for the sGCα-1 subunit normalized for β-actin was set at 100% for NS. Values are expressed as mean ± SEM; *
P<0.05. (
J) mRNA expression of the sGCα-1 subunit correlates with lung function in smokers and patients with COPD. sGC mRNA levels of α-1 subunit of human lung tissues were correlated with forced expiratory volume in 1 second (FEV
1%) (% of predicted). Spearman correlation coefficient (
rs) and
P-value are shown. Figures
G–J have been reprinted with permission of the American Thoracic Society. Copyright © 2016 American Thoracic Society. Glynos C, Dupont LL, Vassilakopoulos T, et al. The role of soluble guanylyl cyclase in chronic obstructive pulmonary disease.
Am J Respir Crit Care Med. 2013;188:789–799.
Citation25 The American Journal of Respiratory and Critical Care Medicine is an Official Journal of the American Thoracic Society.
Abbreviations: TB, tracheal banding; BALF, bronchoalveolar lavage fluid; sGC, soluble guanylyl cyclase; SEM, standard error of the mean; NS, never smokers; S, smokers; wks, weeks.