Figures & data
Table 1 Baseline demographics and patient characteristics of less symptomatic and more symptomatic patients according to baseline E-RS and BDI
Figure 1 One-hour morning postdose FEV1 change from baseline in less symptomatic and more symptomatic patients with COPD at week 24.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate 12 µg; LS, least squares; ITT, intent-to-treat; SE, standard error.
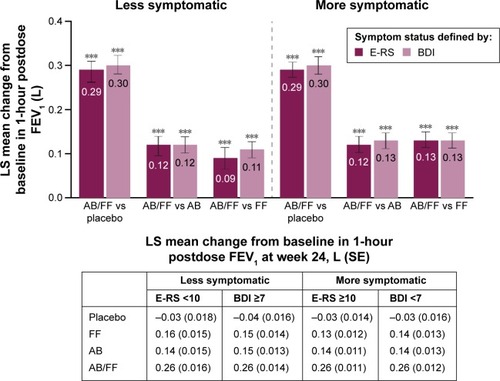
Figure 2 Trough FEV1 (1-hour morning predose) change from baseline in less symptomatic and more and symptomatic patients with COPD at week 24.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FEV1, forced expiratory volume in 1 second; FF, formoterol fumarate 12 µg; ITT, intent-to-treat; LS, least squares; SE, standard error.
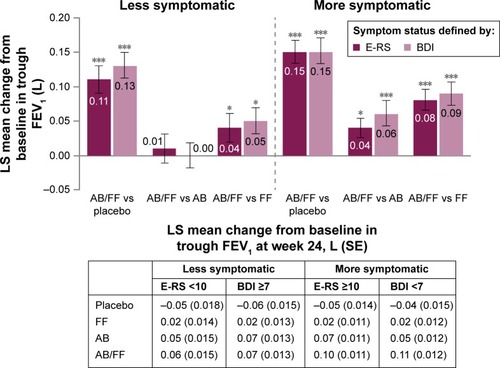
Figure 3 TDI focal score change from baseline in less symptomatic and more symptomatic patients with COPD at week 24.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FF, formoterol fumarate 12 µg; ITT, intent-to-treat; LS, least squares; SE, standard error; TDI, Transition Dyspnea Index.
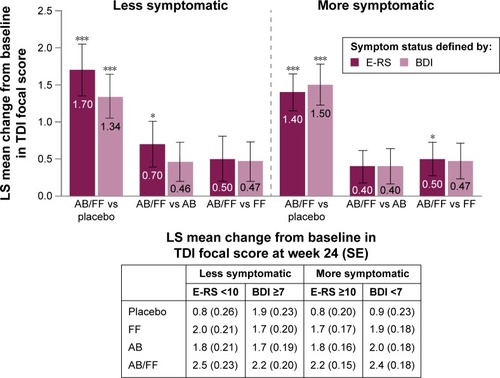
Figure 4 E-RS total score change from baseline in less symptomatic and more symptomatic patients with COPD at week 24.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FF, formoterol fumarate 12 µg; ITT, intent-to-treat; LS, least squares; SE, standard error.
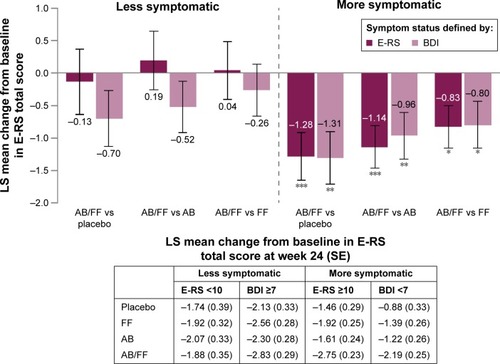
Figure 5 Change from baseline in (A) early-morning and (B) nighttime symptom severity in less symptomatic and more symptomatic patients with COPD at week 24.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FF, formoterol fumarate 12 µg; ITT, intent-to-treat; LS, least squares; SE, standard error.
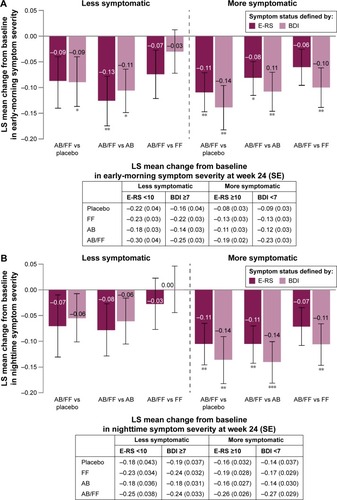
Figure 6 Change from baseline in early-morning limitation of activity in (A) the overall pooled population and (B) less symptomatic and more symptomatic patients with COPD.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FF, formoterol fumarate 12 µg; ITT, intent-to-treat; LS, least squares; SE, standard error.
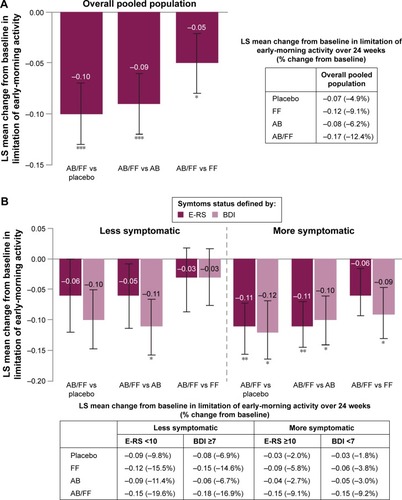
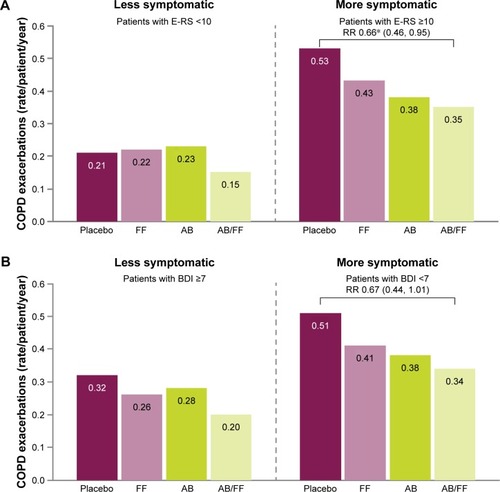
Figure 7 Rate of moderate or severe COPD exacerbations (HCRU criteria) in less symptomatic and more symptomatic patients at Week 24, (A) less symptomatic and more symptomatic defined by E-RS score, (B) less symptomatic and more symptomatic defined by BDI score.
Abbreviations: AB, aclidinium bromide 400 µg; BDI, Baseline Dyspnea Index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; E-RS, Evaluating Respiratory Symptoms; FF, formoterol fumarate 12 µg; HCRU, healthcare resource utilization; RR, rate ratio.