Figures & data
Table 1 Patient demographics and baseline characteristics
Figure 1 Change from baseline in pre-dose trough FEV1 at week 26.
Abbreviations: FEV1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IND/GLY, indacaterol/glycopyrronium 110 μg/50 μg once daily; n, number of patients; SFC, salmeterol/fluticasone 50 μg/500 μg twice daily.
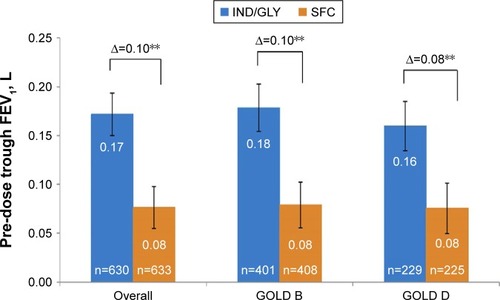
Figure 2 Change from baseline in FEV1 AUC0–12 hours at week 26.
Abbreviations: FEV1 AUC0–12 hours, area under the curve for forced expiratory volume in one second from 0 to 12 hours; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IND/GLY, indacaterol/glycopyrronium 110 μg/50 μg once daily; n, number of patients; SFC, salmeterol/fluticasone 50 μg/500 μg twice daily.
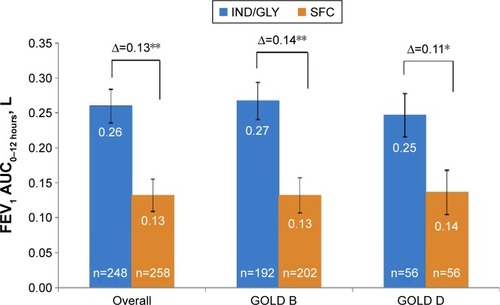
Table 2 Comparison of secondary efficacy outcomes at week 26
Figure 3 Annualized rate of moderate/severe exacerbations during the 26-week treatment period.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; IND/GLY, indacaterol/glycopyrronium 110 μg/50 μg once daily; SFC, salmeterol/fluticasone 50 μg/500 μg twice daily.
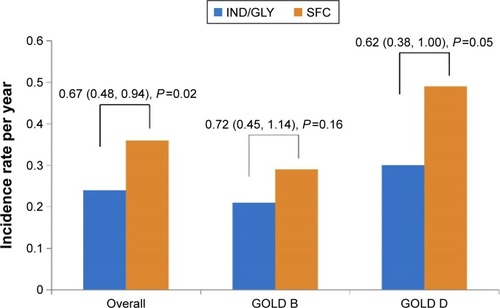
Figure 4 Time to first moderate/severe exacerbation during the 26-week treatment period: (A) patients in the GOLD B subgroup and (B) patients in the GOLD D subgroup.
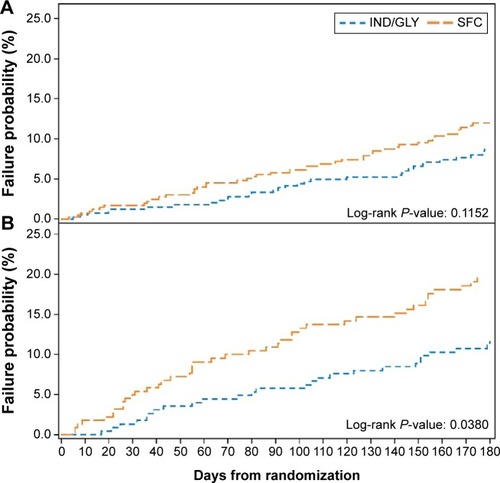
Table 3 AEs, serious AEs, and deaths (safety set)
