Figures & data
Figure 1 Flowchart of EAP identification.
Abbreviations: COPD, chronic obstructive pulmonary disease; EAP, efficacy analyzable population; FEV1, forced expiratory volume in 1 second.
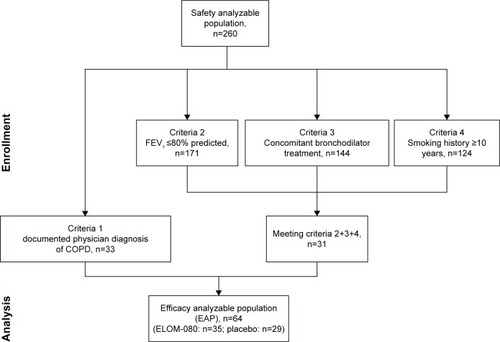
Table 1 Demographic data and FEV1 (%)
Figure 2 Proportion of the subjects with at least one exacerbation after 6 months of treatment.
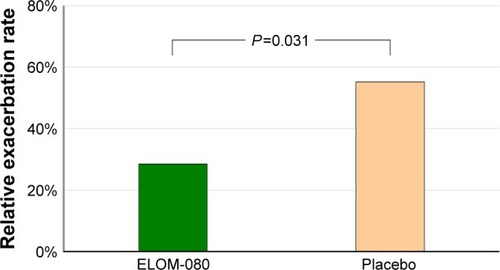
Figure 3 Cumulative numbers of exacerbations during the treatment period.
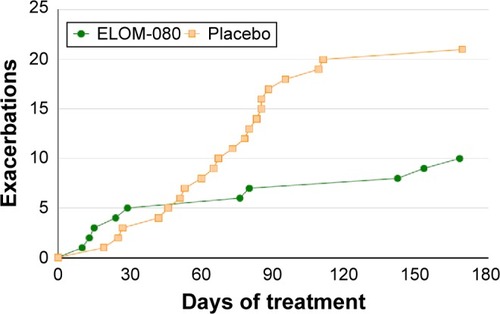
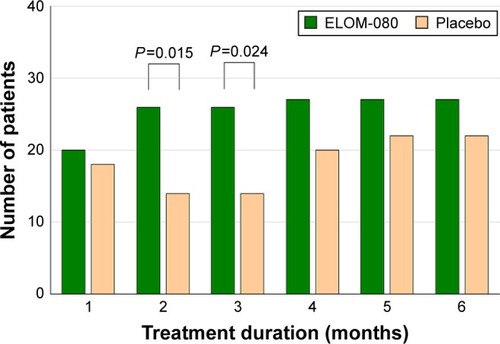
Figure 4 Number of patients without clinical impairment of cough and sputum.
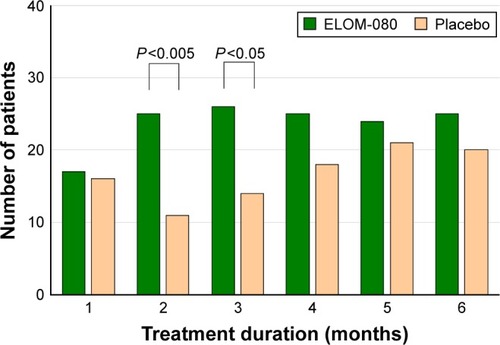
Figure 5 Number of patients with a good or very good health status.