Figures & data
Table 1 RCTs reporting on the CV and cerebrovascular safety of LAMA/LABA combination therapy
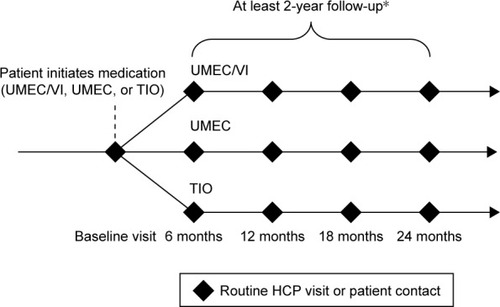
Table 2 Primary and secondary study safety outcomes in the 201038 Post-authorization Safety Study of UMEC/VI combination therapy