Figures & data
Figure 1 Change in SGRQ scores.
Abbreviations: LS, least squares; SGRQ, St George’s Respiratory Questionnaire.
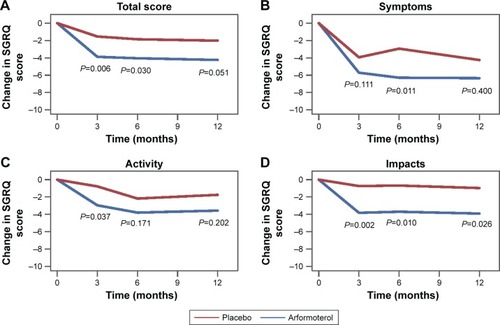
Figure 2 SGRQ Total score change by percentage change in FEV1.
Abbreviations: FEV1, forced expiratory volume in 1 second; SGRQ, St George’s Respiratory Questionnaire.
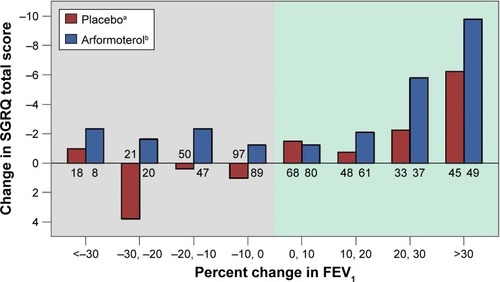
Figure 3 Growth curves of SGRQ Symptoms scores for the two-class solution.
Abbreviations: GMM, growth mixture model; SGRQ, St George’s Respiratory Questionnaire.
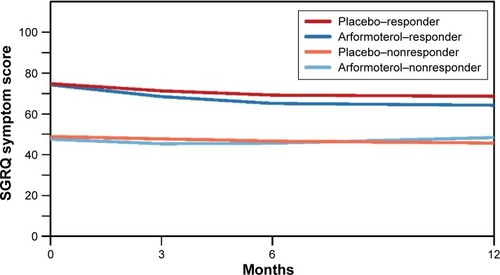
Table 1 Descriptive statistics for baseline characteristics by SGRQ symptoms latent class responder status
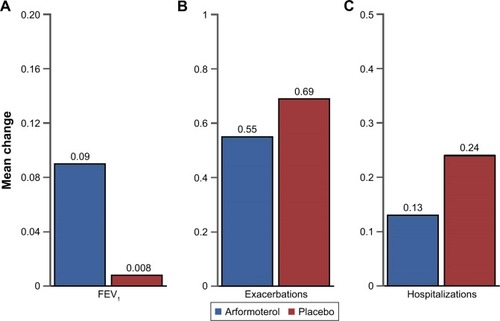
Figure 4 Change in mean FEV1, exacerbations, and hospitalizations for latent class responders treated with arformoterol versus latent class responders treated with placebo.
Abbreviation: FEV1, forced expiratory volume in 1 second.